Abstract
Although the ability to induce with organ-specific antigens autoimmune inflammatory lesions in the brain and thyroid is well-established, it has not been accomplished for the gastrointestinal system. Therefore, purified rat intestinal glycoproteins (RGCG and RCG) with defined biochemical and immunological properties and known to be organ-specific, were employed to study immune responses in four highly inbred strains of rats. Animals subcutaneously immunized with RGCG/RCG (or saline in controls) and Mycobacterium butyricum as the primary adjuvant were followed weekly for (a) weight loss and diarrhoea; (b) development of specific antibody; (c) cell-mediated cytotoxicity by a 51Cr release assay; (d) pathological changes (graded I—IV) in intestine at the time of serial killing; and (e) increase in lamina propria cell count in histological sections taken from both macroscopically normal and abnormal bowel wall.
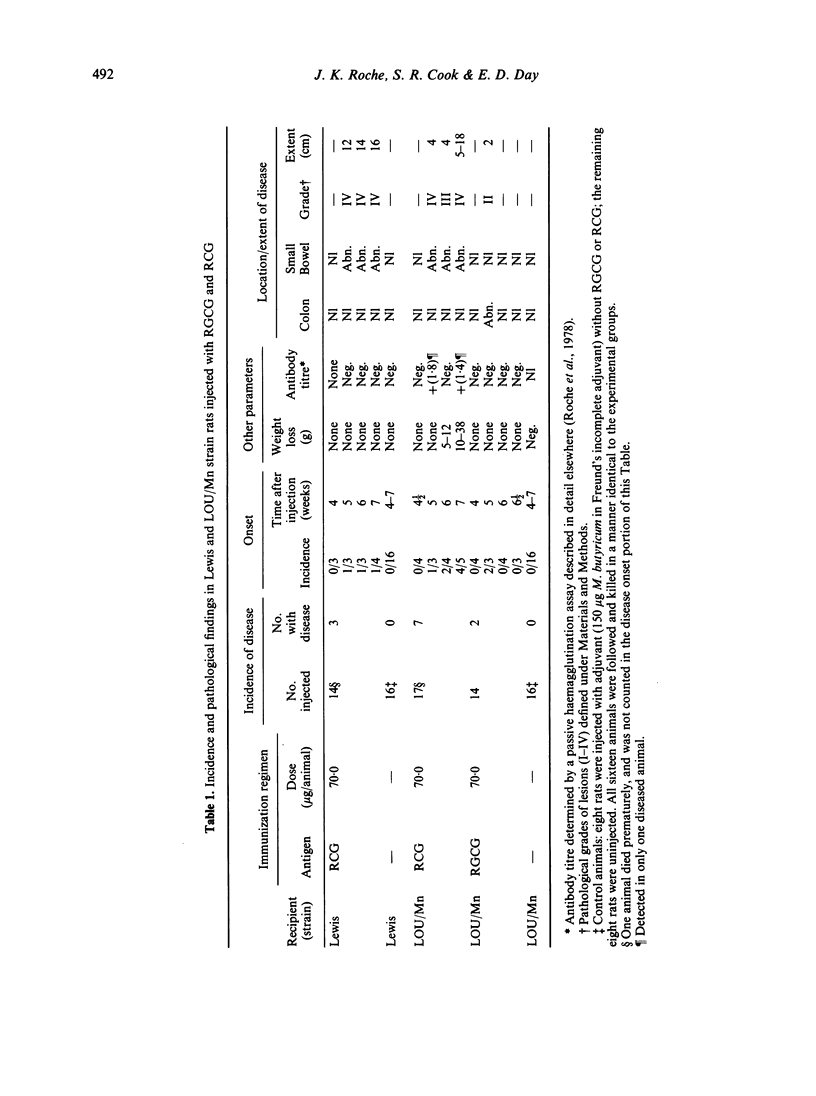
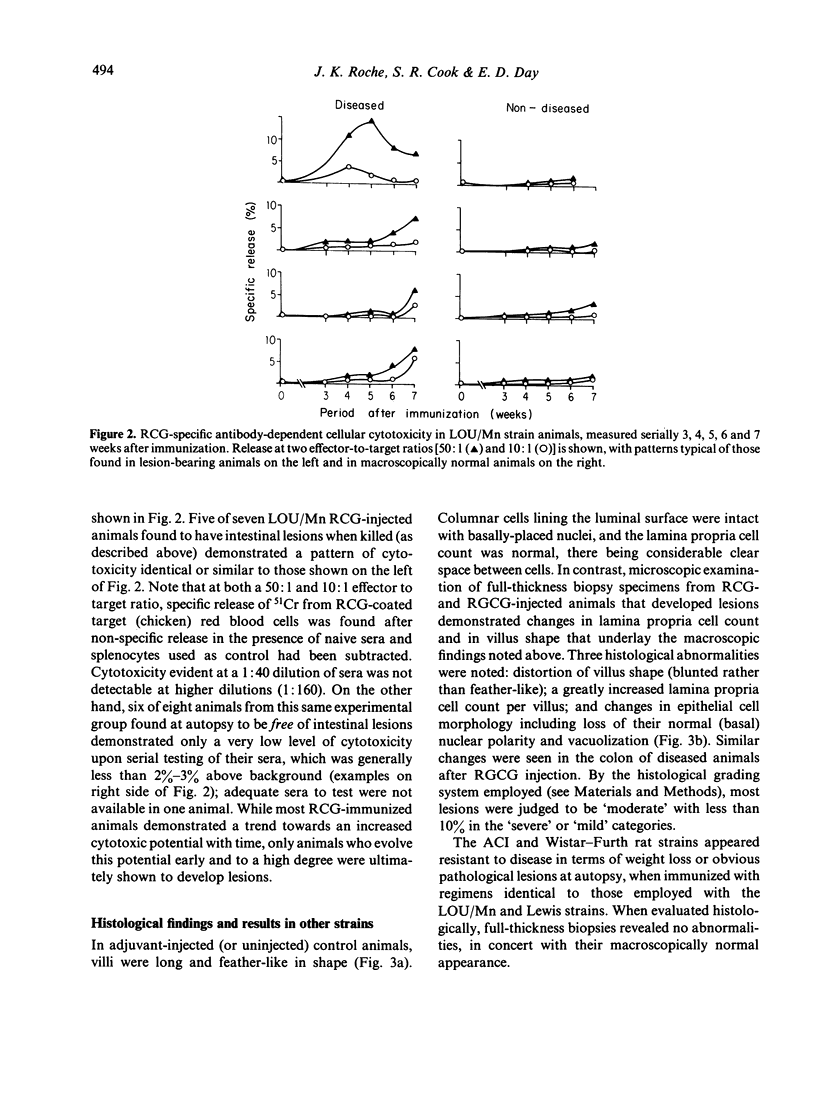
Disease incidence was highest in LOU/Mn strain rats injected with RCG, where small bowel lesions began at the fifth week after immunization (one of three animals), their frequency progressively increasing through the seventh week (four of five animals) when weight loss was most marked. Lewis strain rats injected with RCG had similar small bowel lesions at the sixth and seventh week. Colonic lesions were found in LOU/Mn strain rats injected with RGCG. Antibody-dependent cellular cytotoxic responses to RCG were detectable at both 50:1 and 10:1 effector to target ratios, occurred principally in injected animals who progressed to disease, and were correlated in time with the onset of lesions (weeks 5–7). Pathologically, all small bowel lesions were grade III (dark red granular mucosa) or IV (granular with obvious haemorrhage). Involved segments were 4–16 cm long, confined to the ileum, and diffuse without punctate ulcers. Colonic lesions were 2 cm long and diffusely hyperemic (grade II). Histologically, sections from diseased animals showed three changes: distortion (blunting) of villus shape, a hypercellular lamina propria (grade, moderate) and disruption of columnar epithelial lining cells. Two (ACI and Wistar—Furth) of the four strains studied were non-responders with respect to disease induction. We conclude that purified organ-specific gut glycoproteins can induce bowel wall pathological changes and antibody-dependent cellular cytotoxic reactions in susceptible strains of immunized rats. This technique may be useful for studying both the origin of, and the mediators for, autoimmune disease of the intestine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anver M. R., Cohen J. Animal model of human disease. Ulcerative colitis. Animal model: Ulcerative colitis induced in guinea pigs with degraded carrageenan. Am J Pathol. 1976 Aug;84(2):431–434. [PMC free article] [PubMed] [Google Scholar]
- Askenase P. W., Boone W. T., Binder H. J. Colonic basophil hypersensitivity. J Immunol. 1978 Jan;120(1):198–301. [PubMed] [Google Scholar]
- BROBERGER O., PERLMANN P. Autoantibodies in human ulcerative colitis. J Exp Med. 1959 Nov 1;110:657–674. doi: 10.1084/jem.110.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazin H., Beckers A., Querinjean P. Three classes and four (sub)classes of rat immunoglobulins: IgM, IgA, IgE and IgG1, IgG2a, IgG2b, IgG2c. Eur J Immunol. 1974 Jan;4(1):44–48. doi: 10.1002/eji.1830040112. [DOI] [PubMed] [Google Scholar]
- Bazin H., Querinjean P., Beckers A., Heremans J. F., Dessy F. Transplantable immunoglobulin-secreting tumours in rats. IV. Sixty-three IgE-secreting immunocytoma tumours. Immunology. 1974 Apr;26(4):713–723. [PMC free article] [PubMed] [Google Scholar]
- Day E. D., Varitek V. A., Paterson P. Y. Myelin basic protein serum factor (MBP-SF) in adult Lewis rats: a method for detection and evidence that MBP-SF influences the appearance of antibody to MBP in animals developing experimental allergic encephalomyelitis. Immunochemistry. 1978 Jul;15(7):437–442. doi: 10.1016/0161-5890(78)90071-8. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Paterson P. Y., Day E. D., Varitek V. A. Myelin basic protein serum factor. An endogenous neuroantigen influencing development of experimental allergic encephalomyelitis in Lewis rats. J Exp Med. 1978 Dec 1;148(6):1716–1721. doi: 10.1084/jem.148.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Weigle W. O. Cellular events in the induction of experimental allergic encephalomyelitis in rats. J Exp Med. 1976 Sep 1;144(3):604–616. doi: 10.1084/jem.144.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATERSON P. Y. Transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med. 1960 Jan 1;111:119–136. doi: 10.1084/jem.111.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J. K., Day E. D., Hill H. D. Rabbit antibodies to ovine-submaxillary mucin. Detection, specificity and cross-reactivity. Immunochemistry. 1978 May;15(5):339–343. doi: 10.1016/0161-5890(78)90096-2. [DOI] [PubMed] [Google Scholar]
- Roche J. K., Varitek V. A., Hill H. D., Day E. D. Specificity and T-lymphocyte dependence of the humoral immune response in the rat to purified ovine and porcine mucins. Mol Immunol. 1979 Aug;16(8):609–619. doi: 10.1016/0161-5890(79)90124-x. [DOI] [PubMed] [Google Scholar]
- SHEAN F. C., BARKER W. F., FONKALSRUD E. W. STUDIES ON ACTIVE AND PASSIVE ANTIBODY INDUCED COLITIS IN THE DOG. Am J Surg. 1964 Feb;107:337–339. doi: 10.1016/0002-9610(64)90277-6. [DOI] [PubMed] [Google Scholar]
- Sharp G. C., Mullen H., Kyriakos M. Production of augmented experimental autoimmune thyroiditis lesions by combined transfer of antiserum and lymph node cells. J Immunol. 1974 Feb;112(2):478–487. [PubMed] [Google Scholar]
- Stobo J. D., Tomasi T. B., Huizenga K. A., Spencer R. J., Shorter R. G. In vitro studies of inflammatory bowel disease. Surface receptors of the mononuclear cell required to lyse allogeneic colonic epithelial cells. Gastroenterology. 1976 Feb;70(2):171–176. [PubMed] [Google Scholar]
- Talal N., Dauphinee M., Pillarisetty R., Goldblum R. Effect of thymosin on thymocyte proliferation and autoimmunity in NZB mice. Ann N Y Acad Sci. 1975 Feb 28;249:438–450. doi: 10.1111/j.1749-6632.1975.tb29092.x. [DOI] [PubMed] [Google Scholar]
- Vladutiu A. O., Rose N. R. Transfer of experimental autoimmune thyroiditis of the mouse by serum. J Immunol. 1971 Apr;106(4):1139–1142. [PubMed] [Google Scholar]
- WITEBSKY E., ROSE N. R., NADEL H. Studies on organ specificity X. The serologic specificity of pancreas extracts. J Immunol. 1960 Dec;85:568–574. [PubMed] [Google Scholar]
- Watson D. W., Quigley A., Bolt R. J. Effect of lymphocytes from patients with ulcerative colitis on human adult colon epithelial cells. Gastroenterology. 1966 Dec;51(6):985–993. [PubMed] [Google Scholar]
- Williams R. M., Moore M. J. Linkage of susceptibility to experimental allergic encephalomyelitis to the major histocompatibility locus in the rat. J Exp Med. 1973 Oct 1;138(4):775–783. doi: 10.1084/jem.138.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]