Abstract
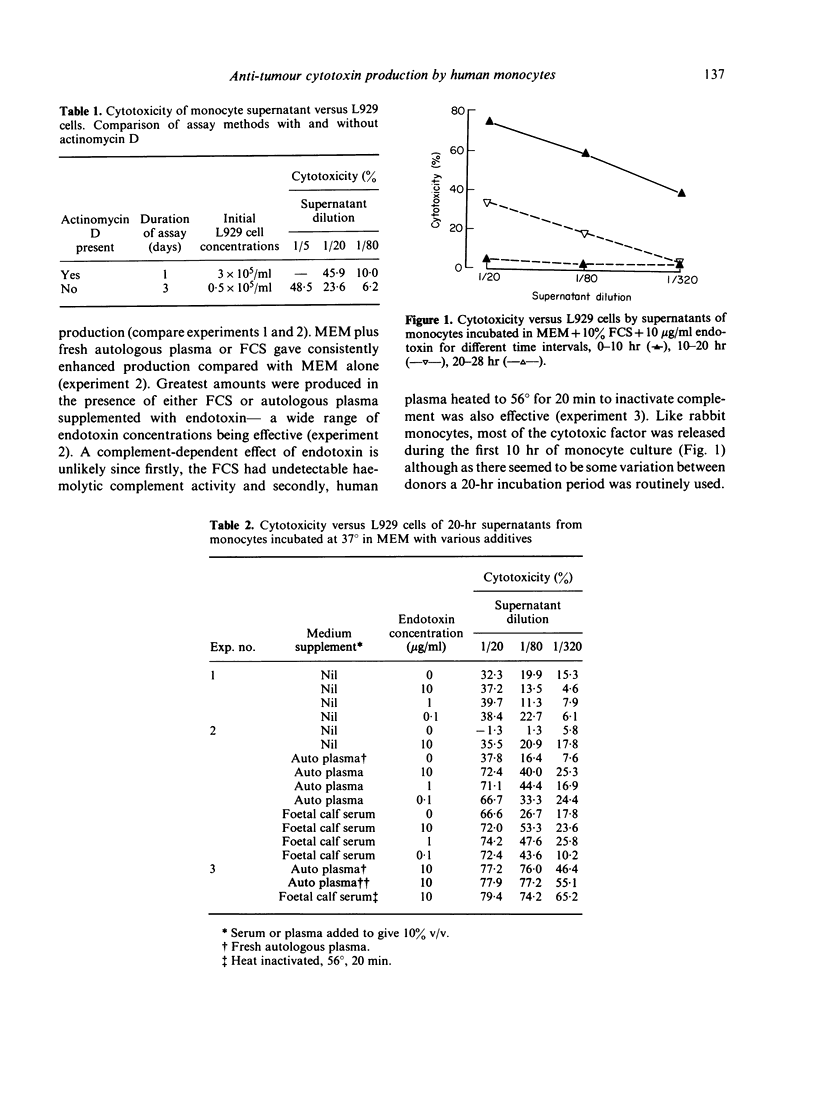
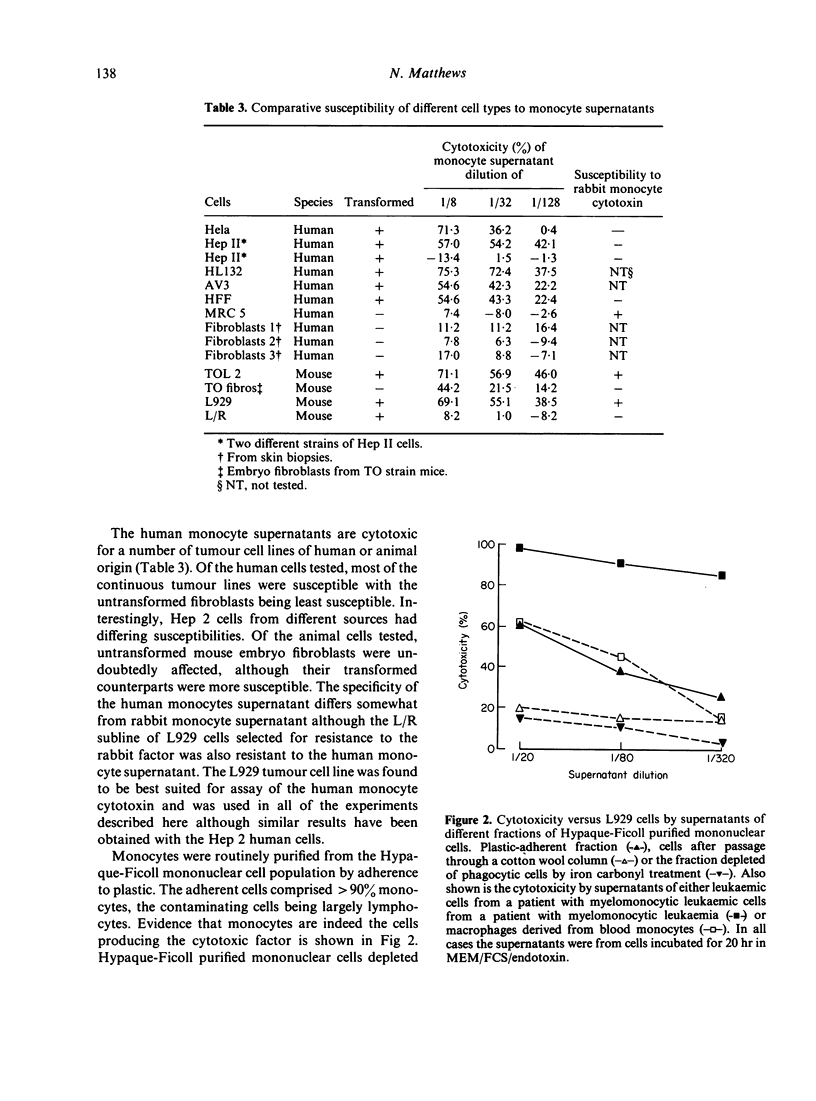
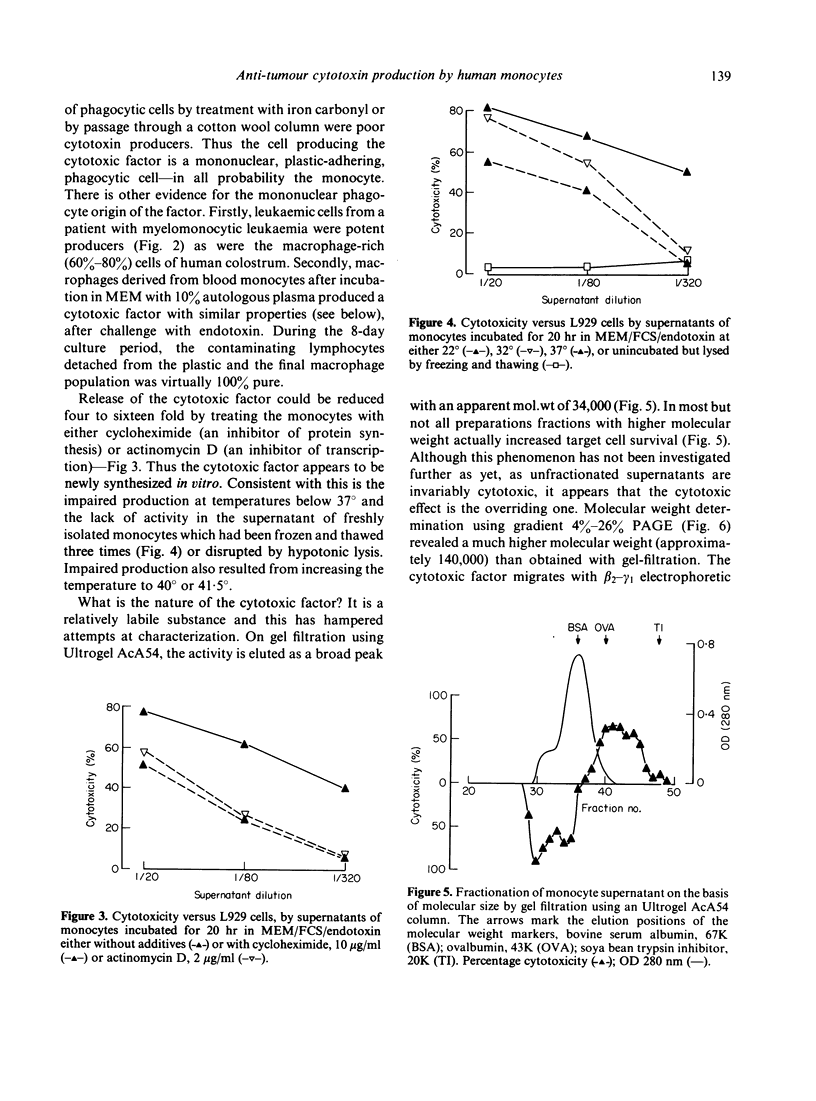
Human monocytes incubated in vitro for 20 hr at 37 degrees release a factor which is cytotoxic to a number of human and murine tumour cell lines: untransformed cells appear to be less susceptible. A similar factor is produced under comparable conditions by myelomonocytic leukaemic cells and by macrophages derived from monocytes by in vitro culture for 8 days. Maximum production of the factor occurred in the presence of foetal calf serum or autologous plasma and endotoxin. The factor is newly synthesized in culture as its production is reduced if the monocytes are treated with cycloheximide or actinomycin D or incubated at lower temperatures. Freshly isolated monocytes do not release the factor on freeze--thaw or hypotonic lysis. The monocyte cytotoxin has apparent molecular weights of 34,000 on Ultrogel AcA54 gel filtration and 140,000 on gradient polyacrylamide gel electrophoresis; it has beta 2--gamma 1 electrophoretic mobility in polyacrylamide gel and does not appear to be C3a or arginase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton S., Perlmann H., Perlmann P. Thymus-dependent and thymus-independent effector functions of mouse lymphoid cells. Comparison of cytotoxicity and primary antibody formation in vitro. Cell Immunol. 1973 Sep;8(3):420–434. doi: 10.1016/0008-8749(73)90133-0. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Ferluga J., Schorlemmer H. U., Baptista L. C., Allison A. C. Production of the complement cleavage product, C3a, by activated macrophages and its tumorolytic effects. Clin Exp Immunol. 1978 Mar;31(3):512–517. [PMC free article] [PubMed] [Google Scholar]
- Goodman M. G., Weigle W. O., Hugli T. E. Inability of the C3a anaphylatoxin to promote cellular lysis. Nature. 1980 Jan 3;283(5742):78–80. doi: 10.1038/283078a0. [DOI] [PubMed] [Google Scholar]
- Hammerström J., Unsgaard G., Lamvik J. Activation of human monocytes by mediators from lymphocytes stimulated with Corynebacterium parvum. Acta Pathol Microbiol Scand C. 1979 Jun;87C(3):167–175. [PubMed] [Google Scholar]
- Hibbs J. B., Jr Discrimination between neoplastic and non-neoplastic cells in vitro by activated macrophages. J Natl Cancer Inst. 1974 Nov;53(5):1487–1492. doi: 10.1093/jnci/53.5.1487. [DOI] [PubMed] [Google Scholar]
- Holtermann O. A., Klein E., Casale G. P. Selective cytotoxicity of peritoneal leucocytes for neoplastic cells. Cell Immunol. 1973 Dec;9(3):339–352. doi: 10.1016/0008-8749(73)90049-x. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Kight N., Temple A., Allison A. C. Spontaneous and induced cytotoxic properties of human adherent mononuclear cells: killing of non-sensitized and antibody-coated non-erythroid cells. Immunology. 1979 Feb;36(2):221–228. [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Maclaurin B. P. "Spontaneous" cytolysis by normal human lymphocytes of Burkitt's lymphoma cells of the EB2 cell line. Aust J Exp Biol Med Sci. 1974 Aug;52(4):655–661. doi: 10.1038/icb.1974.64. [DOI] [PubMed] [Google Scholar]
- Matthews N., Ryley H. C., Neale M. L. Tumour-necrosis factor from the rabbit. IV. Purification and chemical characterization. Br J Cancer. 1980 Sep;42(3):416–422. doi: 10.1038/bjc.1980.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N. Tumour-necrosis factor from the rabbit. II. Production by monocytes. Br J Cancer. 1978 Aug;38(2):310–315. doi: 10.1038/bjc.1978.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. P., Lucas Z. J. Cytotoxic activity of lymphocytes. V. Role of soluble toxin in macrophage-inhibited cultures of tumor cells. J Immunol. 1975 Aug;115(2):395–404. [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]