Abstract
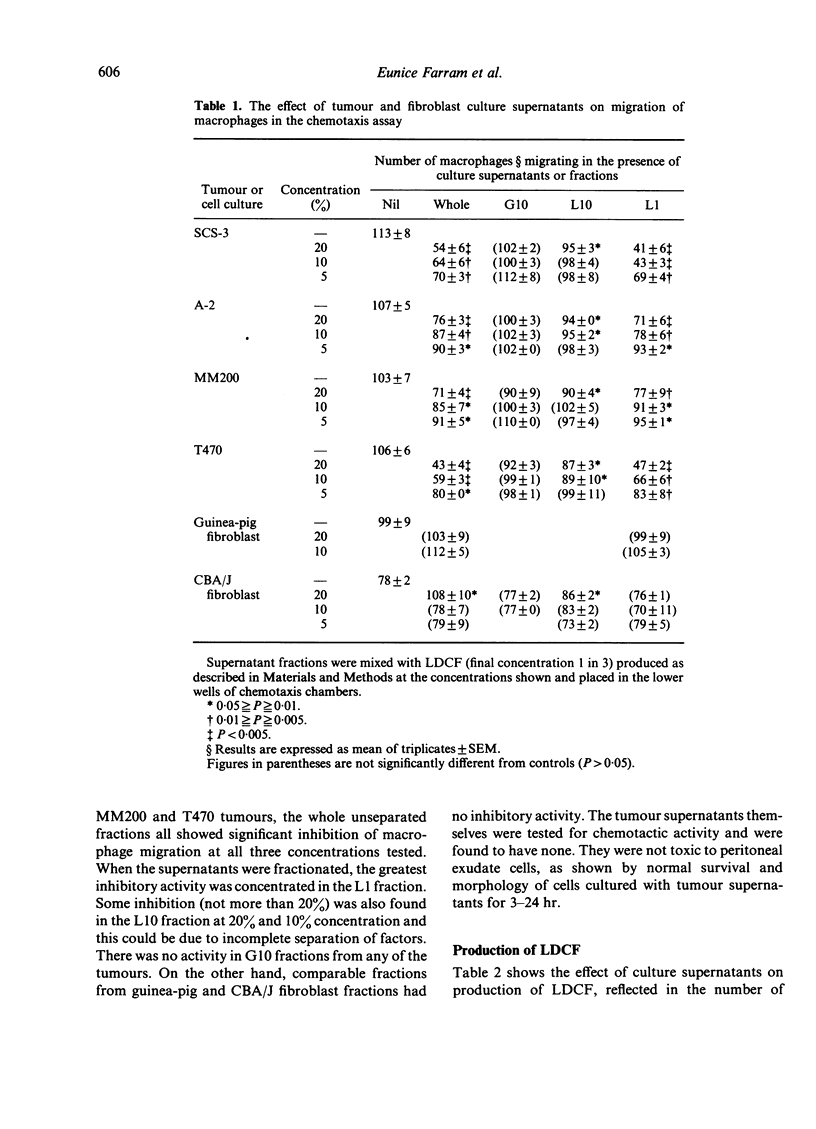
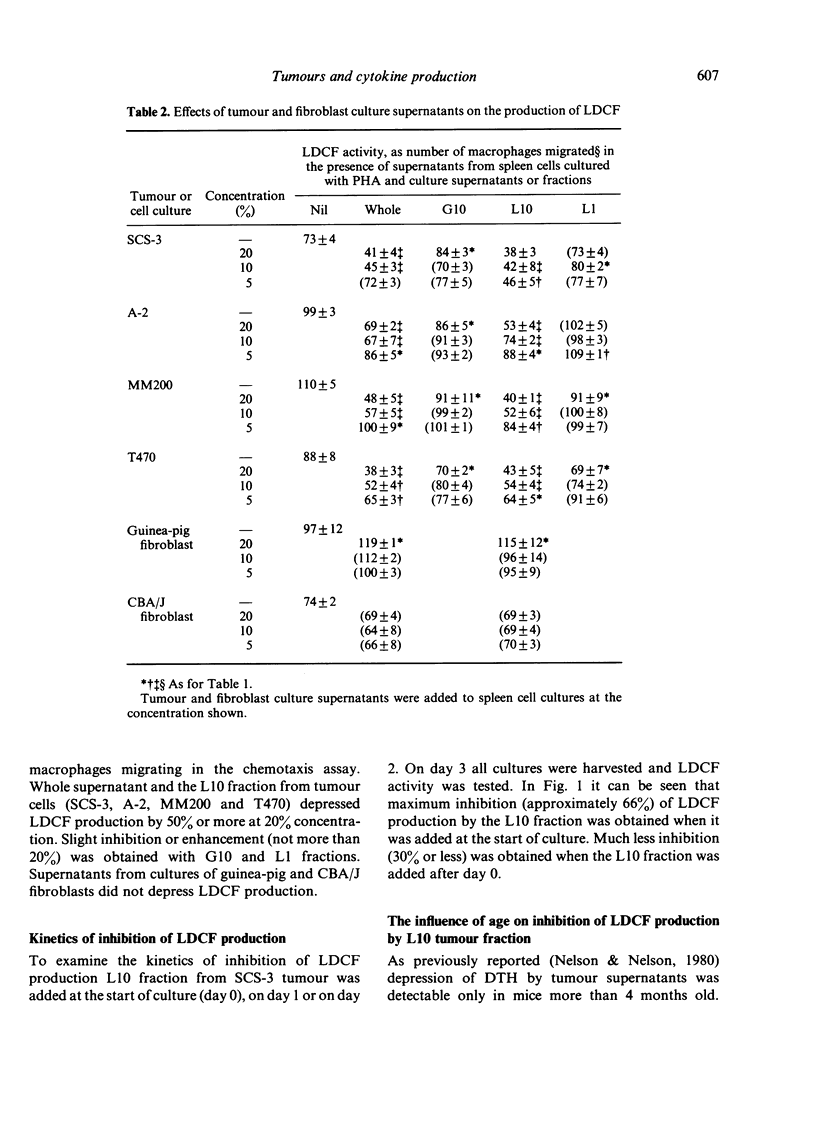
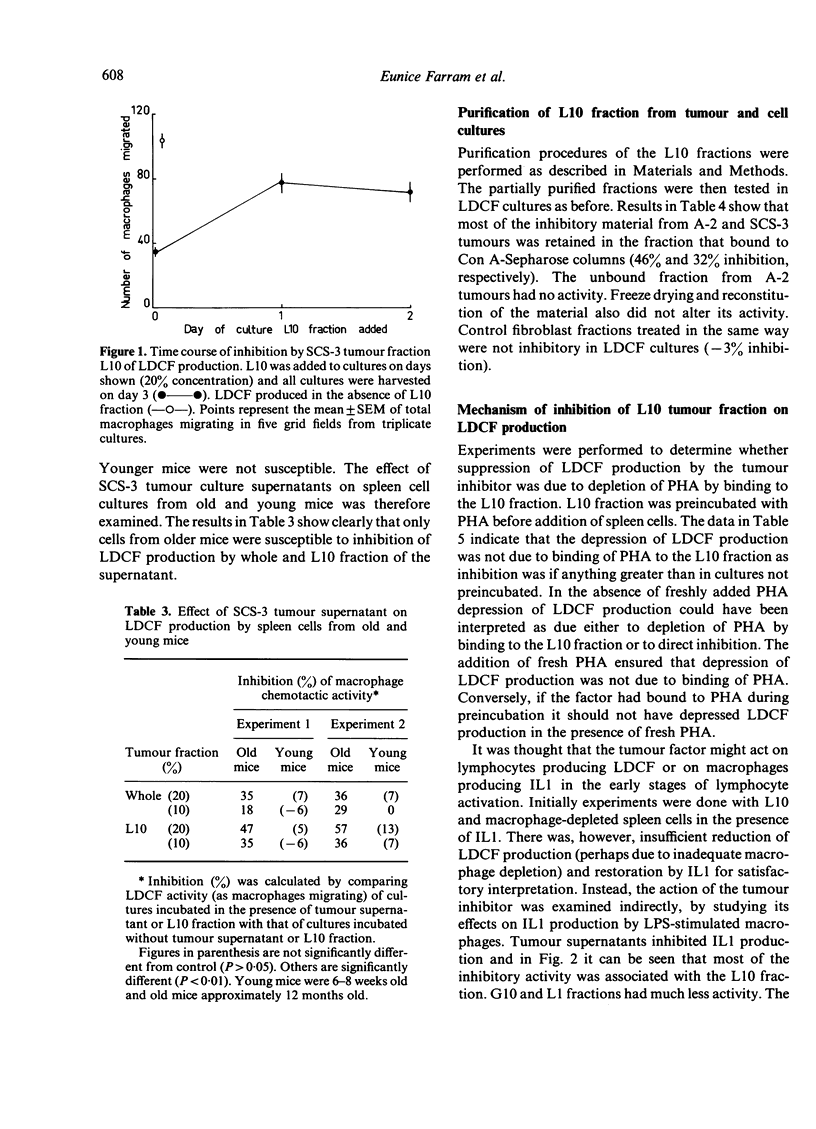
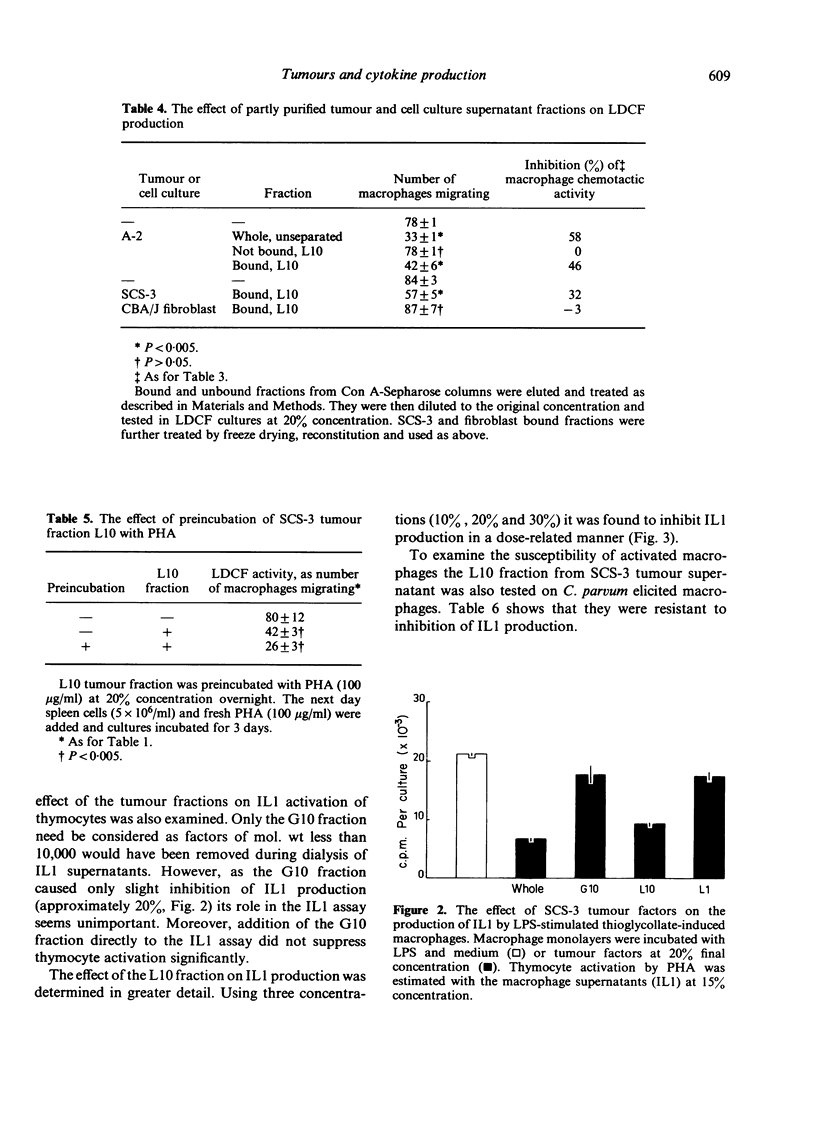
Supernatants from cultured mouse and human tumour cells, but not mouse or guinea-pig fibroblasts, inhibited the production of a lymphokine, macrophage chemotactic factor, by PHA-stimulated mouse spleen cells. The supernatants affected spleen cells from old, but not young, mice. They were most active if added at the start of the spleen cell culture and did not act by binding phytohaemagglutinin (PHA). The active material had an approximate molecular weight, on membrane filtration, of 1000-10,000 and could be bound to and eluted from Con A-Sepharose. Tumour supernatant factor(s) of similar molecular weight inhibited the production of interleukin 1 (lymphocyte activating factor) in response to lipopolysaccharide by stimulated thioglycollate-induced peritoneal exudate macrophages, but not by Corynebacterium parvum-activated macrophages. Similar tumour-produced material has been found to inhibit the early phase of delayed-type hypersensitivity reactions in older mice. It is suggested that this effect is due, at least in part, to inhibition of interleukin 1 production leading to inhibition of lymphokine production.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Farram E., Nelson D. S. Mechanism of action of mouse macrophages as antitumor effector cells: role of arginase. Cell Immunol. 1980 Oct;55(2):283–293. doi: 10.1016/0008-8749(80)90161-6. [DOI] [PubMed] [Google Scholar]
- Farram E., Nelson M., Nelson D. S. Macrophages and resistance to tumors. The effects of tumor cell products on monocytopoiesis. J Reticuloendothel Soc. 1981 Oct;30(4):259–269. [PubMed] [Google Scholar]
- Fauve R. M., Hevin B., Jacob H., Gaillard J. A., Jacob F. Antiinflammatory effects of murine malignant cells. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4052–4056. doi: 10.1073/pnas.71.10.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Chapman H. A., Jr, Weinberg J. B. The macrophage as an antineoplastic surveillance cell: biological perspectives. J Reticuloendothel Soc. 1978 Nov;24(5):549–570. [PubMed] [Google Scholar]
- Kearney R., Nelson D. S. Concomitant immunity to syngeneic methylcholanthrene-induced tumours in mice. Occurrence and specificity of concomitant immunity. Aust J Exp Biol Med Sci. 1973 Dec;51(6):723–735. doi: 10.1038/icb.1973.70. [DOI] [PubMed] [Google Scholar]
- Matsubara S., Suzuki M., Nakamura M., Edo K., Ishida N. Isolation of an inhibitor of type II interferon induction from tumor ascitic fluids. Cancer Res. 1980 Jul;40(7):2534–2538. [PubMed] [Google Scholar]
- Meltzer M. S. Chemotactic response of mouse macrophages to culture fluids mitogen-stimulated spleen cells. Clin Immunol Immunopathol. 1976 Sep;6(2):238–247. doi: 10.1016/0090-1229(76)90115-x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Ben-Zvi A. Studies on the role of lymphocyte-activating factor (Interleukin 1) in antigen-induced lymph node lymphocyte proliferation. Cell Immunol. 1980 Sep 1;54(2):382–389. doi: 10.1016/0008-8749(80)90218-x. [DOI] [PubMed] [Google Scholar]
- Nelson D. S., Nelson M., Farram E., Inoue Y. Cancer and subversion of host defences. Aust J Exp Biol Med Sci. 1981 Jun;59(Pt 3):229–262. doi: 10.1038/icb.1981.18. [DOI] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. Macrophages and resistance to tumors. IV. Influence of age on susceptibility of mice to anti-inflammatory and antimacrophage effects of tumor cell products. J Natl Cancer Inst. 1980 Oct;65(4):781–789. doi: 10.1093/jnci/65.4.781. [DOI] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. Macrophages and resistance to tumours. I. Inhibition of delayed-type hypersensitivity reactions by tumour cells and by soluble prducts affecting macrophages. Immunology. 1978 Feb;34(2):277–290. [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Depression of macrophage function by a factor produced by neoplasms: a merchanism for abrogation of immune surveillance. J Immunol. 1976 Oct;117(4):1243–1249. [PubMed] [Google Scholar]
- Wahl S. M., Wilton J. M., Rosenstreich D. L., Oppenheim J. J. The role of macrophages in the production of lymphokines by T and B lymphocytes. J Immunol. 1975 Apr;114(4):1296–1301. [PubMed] [Google Scholar]
- Wells J. H., Cain W. A., Wells R. S., Bozalis J. R. Suppression of tuberculin and phytohemagglutinin skin tests by large tumors in inbred mice. Int Arch Allergy Appl Immunol. 1974;47(3):362–368. doi: 10.1159/000231229. [DOI] [PubMed] [Google Scholar]


