Figure 3. 17β-estradiol induced homo-dimerization of the ERα as assessed by BRET2 .
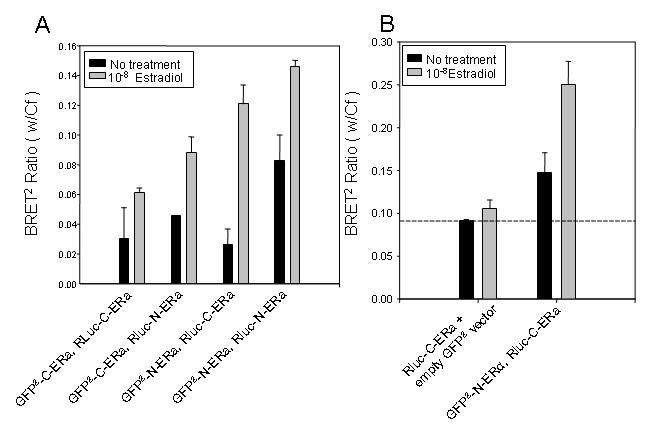
HEK-293 cells were transiently co-expressed with the GFP2-C-ERα (40µg) and Rluc-C-ERα (10µg) fusion proteins to generate a dose-response curve with estradiol treatment (data not shown). The estradiol-induced interaction by BRET2 occurs between all combinations of ERα fusion proteins, regardless of differences in tertiary structure. HEK-293 cells were transfected with 10µg each of a GFP2-ERα and Rluc-ERα fusion protein and incubated with vehicle or 10-8 M estradiol for 30 minutes (A). Luminescence and fluorescence emissions were then read immediately following addition of DeepBlueC substrate for the BRET2 assay. To determine the ligand-dependent and -independent homodimerization of ERα, HEK-293 were transfected with 10µg of Rluc-C-ERα and 10µg of either GFP2-C-ERα or the GFP2-C vector alone and treated for 30 minutes with 10-8M estradiol (B). The ligand-dependent and -independent BRET2 interaction of GFP2-C-ERα and Rluc-C-ERα with and without estradiol treatment is indicated by bars above the dashed line. The dashed line represents basal activity. This figure is representative of five experiments.
