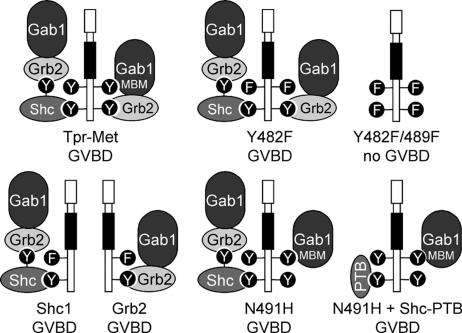
Figure 1.
Tpr-Met mutants that induce GVBD have the capacity to recruit Gab1. Diagram displays the mechanism by which each mutant of the Tpr-Met oncoprotein is predicted to recruit the Gab1 protein and whether these Tpr-Met oncoproteins induce GVBD (Mood et al., 2006): Tpr-Met, wt Tpr-Met oncoprotein, which interacts with Gab1 via direct and indirect Grb2-dependent mechanisms (Lock et al., 2003); Y482F, mutant of Tpr-Met where Tyr-482 is replaced with Phe (Fixman et al., 1996) and cannot bind directly to Gab1 but retains a Grb2-dependent means to associate with Gab1 (Lock et al., 2003); Y482/489F, mutant of Tpr-Met where Tyr-482 and Tyr-489 are replaced with Phe (Fixman et al., 1996), preventing direct and indirect interaction with Gab1; Shc1, Shc-specific docking Tpr-Met variant that interacts with Gab1 via a Grb2-dependent mechanism (Saucier et al., 2002); Grb2, Grb2-specific docking Tpr-Met variant that interacts indirectly with Gab1 (Saucier et al., 2002); N491H, mutant of Tpr-Met where Gln-491 is replaced with His, preventing direct binding of Grb2 (Fixman et al., 1996), but it can interact directly with Gab1 via phosphotyrosine Y482 (Lock et al., 2003); PTB, PTB domain of Shc, which when expressed in cells, blocks the association of Tpr-Met with the adaptor protein Shc (Mood et al., 2006); and Gab1-Grb2–associated binder 1 and MBM-Met receptor binding motif of Gab1 (Lock et al., 2003).