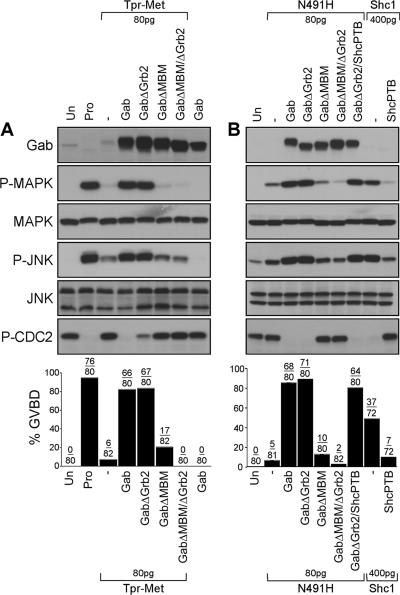
Figure 4.
The Grb2 binding site of Gab1, but not the MBM, is dispensable for the induction of cell cycle progression and activation of MAPK and JNK by the Tpr-Met oncoprotein. (A) Suboptimal levels of Tpr-Met RNA (80 pg/oocyte) were injected in oocytes in the presence or absence of RNAs encoding wt or mutant forms of Gab1 (400 pg/oocyte). Oocytes left uninjected or treated with 5 μM progesterone were used, respectively, as negative and positive controls for GVBD. (B) Oocytes were injected with low levels of the N491H Tpr-Met mutant RNA (80 pg/oocyte) in the presence or absence of the wt or mutant forms of Gab1 RNAs (400 pg/oocyte), or they were singly injected with optimal concentrations of the Tpr-Met Shc-specific docking variant RNA (400 pg/oocyte), in the presence or absence of the dominant-negative ShcPTB RNA (400 pg/oocyte). These data are representative of three independent experiments, and the number of oocytes displaying GVBD over the number of oocytes injected is shown as a percentage in histograms. Lysates prepared from oocytes described above were analyzed by immunoblot conducted with specific antibodies raised against Gab1, phospho-MAPK, MAPK, phospho-JNK, or JNK1/2 or phospho-tyrosine15-Cdc2. GabΔGrb2, mutant of Gab1 with deletion of proline-rich domains (Pro4, aa 337-346/Pro5, aa 517-522) required for Grb2 interaction (Lock et al., 2000); GabΔMBM, mutant of Gab1 where Arg-491 is substituted with Ala, disrupting the MBM essential for direct interaction with the Met receptor (Lock et al., 2003); and GabΔMBMΔGrb2, mutant of Gab1 lacking both the Grb2 binding sites and MBM (Lock et al., 2003).