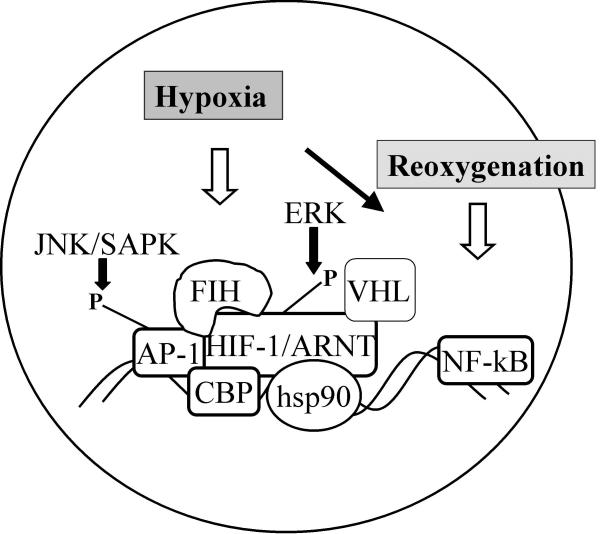
Figure 2.
Hypoxic activation of transcription factors HIF-1α, AP-1 and NF-κB. Cells exposed to hypoxia activate the cellular transcription factors HIF-1α, AP-1 and NF-κB. Under normoxic conditions the von Hippel Lindau tumor suppressor protein mediates ubiquitination and degradation of HIF-1α. This mechanism is inhibited under hypoxia. As a consequence, the HIF-1α protein is stabilized and shows enhanced expression. There are a series of factors that may interfere with HIF-1α under hypoxia, such as cJun/AP-1, heat shock protein (hsp) 90, and the transcriptional co-activator CBP/p300. Further mechanisms of HIF regulation include phosphorylation by extracellular signal regulated kinase (ERK), and phosphorylation of its interaction partner, cJun/AP-1 via stress activated protein kinase, JNK/SAPK. Recently, a factor inhibiting HIF-1α activation, FIH, has been described, representing a further level of HIF regulation. Upon reoxygenation, NF-κB, a well-known transcription factor involved in transcriptional regulation of immune response genes, is activated. However, evidence has been provided that NF-κB activation may also be induced by hypoxia.