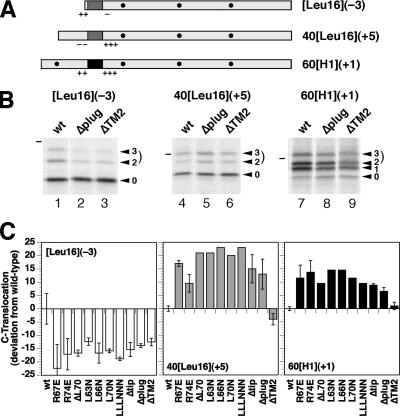
Figure 3.
All plug mutations have the same effect on the topology of model proteins. (A) Diagnostic protein substrates to analyze topology changes are shown schematically with the signal-anchor sequence in dark gray (Leu16) or black (of the asialoglycoprotein receptor H1), flanking charges as + and −, and glycosylation sites as black dots. Their names indicate the length of the N-terminal hydrophilic sequence preceding the signal, the hydrophobic signal core (H1 or Leu16) in brackets, and the charge difference Δ(C–N) according to Hartmann et al. (1989) in parentheses. (B) The three substrates were expressed in Δssh1 cells with wild-type, Δplug, or ΔTM2 Sec61p, pulse labeled for 5 min with [35S]methionine, and analyzed by immunoprecipitation, SDS-gel electrophoresis, and autoradiography. The forms corresponding to the polypeptides with zero, one, two, or three glycans are indicated with arrowheads. The dash designates the position of the 37-kDa molecular weight standard. (C) Effect of indicated Sec61p mutants on the orientation of the model proteins was determined by PhosphorImager quantitation of labeling experiments like those shown in B. Two- and threefold glycosylated species together represent polypeptides with a translocated C terminus. Their fraction of the total (in case of 60[H1](+1) excluding the unglycosylated, not integrated products) are represented as the deviation from the value for the wild-type in percentage points). The results of single determinations (no error bars) or of two to six measurements (average with SD) are shown. The absolute fraction of C-terminally translocated products with wild-type Sec61p was 29.1% for [Leu16](−3), 46.3% for 40[Leu16](+5), and 60.2% for 60[H1](+1).