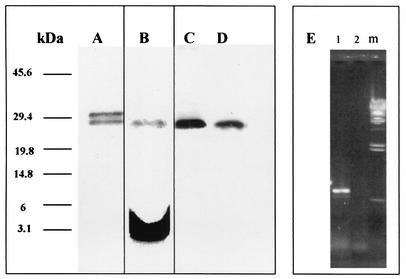
FIG. 1.
Purification and metabolic radiolabeling of the recombinant 27-kDa antigen in E. coli and expression of the native form in BCG and M. tuberculosis. E. coli SG13009 cells harboring the plasmid pRHB27 were grown to an OD600 of 0.7 with or without [3H]palmitic acid. Protein production was induced by IPTG. The induced protein was purified using a nickel-nitrilotriacetic acid column, and samples were analyzed by SDS-PAGE and stained with Coomassie blue. The radioactively labeled samples were exposed to an X-ray film for 1 month. Numbers at the left indicate molecular masses in kilodaltons. Lane A, SDS-PAGE of the purified recombinant 27-kDa lipoprotein; lane B, an autoradiogram of the purified radiolabeled 27-kDa lipoprotein after SDS-PAGE analysis; lanes C and D, Western blot of BCG and M. tuberculosis H37Rv lysates, respectively, exposed to rabbit-anti-27-kDa serum; lane E, RT-PCR of the LprG gene in HeLa cells transfected with pIHB27 (1), pRHB27 as a control vector (2), and λ HindIII DNA marker (m).