Figure 3.
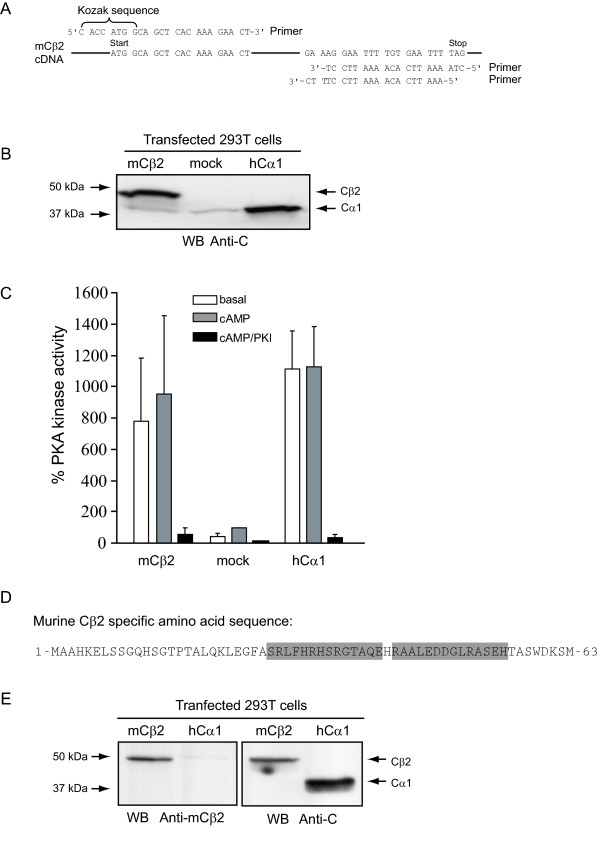
Murine Cβ2 cDNA encodes an active protein kinase of 47 kDa recognized by a Cβ2-specific antiserum. (A) Primers used to amplify mouse Cβ2 cDNA for insertion into pENTR/D-TOPO (Gateway system, Invitrogen). The upper 5'-end primer (upper sequence left) was designed to produce an insert with a Kozak sequence associated with the start codon (ATG). Two different 3'-end primers (lower right two sequences) were designed in order to include or exclude the stop codon (TAG). In the absence of the stop codon, the mouse Cβ2 would be expressed with a C-terminal tag, which is contained in the vector (Gateway, Invitrogen). Mouse Cβ2 cDNA was PCR amplified and subcloned into the pcDNA-DEST40 expression vector to yield pcDNA-DEST40 mCβ2. (B) HEK 293T cells (3.5 × 105 cells/ml) were transfected with 2μg DNA/ml of pcDNA-DEST40mCβ2 (mCβ2), mock-transfected (mock), or transfected with pEF-DEST51hCα1 (hCα1) for 24 h. Cells were lysed and analysed by SDS-PAGE and anti-pan C immunoblotting. Note that mCβ2, but not mock or hCα1 transfected cell extracts, contained an anti-C immunoreactive protein of 47 kDa. All lanes revealed a 40 kDa protein band which was most intense in cells transfected with pEF-DEST51hCα1 . (C) The same lysates were analysed for PKA-specific kinase activity in the absence (empty bars, basal) or presence (gray bars, cAMP) of 5μM cAMP, or both cAMP and PKI (black bars, cAMP/PKI). Activities were calculated relative to the activity of the mock-transfected lysate, which was set to 100 %. Bars represent mean activities of three experiments ± standard deviation. (D) The sequence of the two mCβ2 specific peptides (boxed) used to immunize rabbits to make mCβ2 specific antiserum. (E) Two rabbits were co-immunized with the two peptides and the resulting immune sera tested for immunoreactivity and specificity using cell extracts of HEK 293T cell transfected with mCβ2 (pcDNA-DEST40mCβ2) or hCα1 (pEF-DEST51hCα1). Left panel: The mCβ2 serum recognized a 47 kDa immunoreactive protein in mCβ2 but not in hCα1 transfected cell lysates. No cross-reactivity to human Cα1 could be observed. Right panel: Immunoblotting of the same lysates with a pan anti-C antibody revealed a 47 and a 40 kDa immunoreactive protein in the two extracts respectively, confirming expression of transfected constructs.