Figure 8. A link between the fingers subdomain and conserved structural motif A of the palm subdomain of PV 3Dpol.
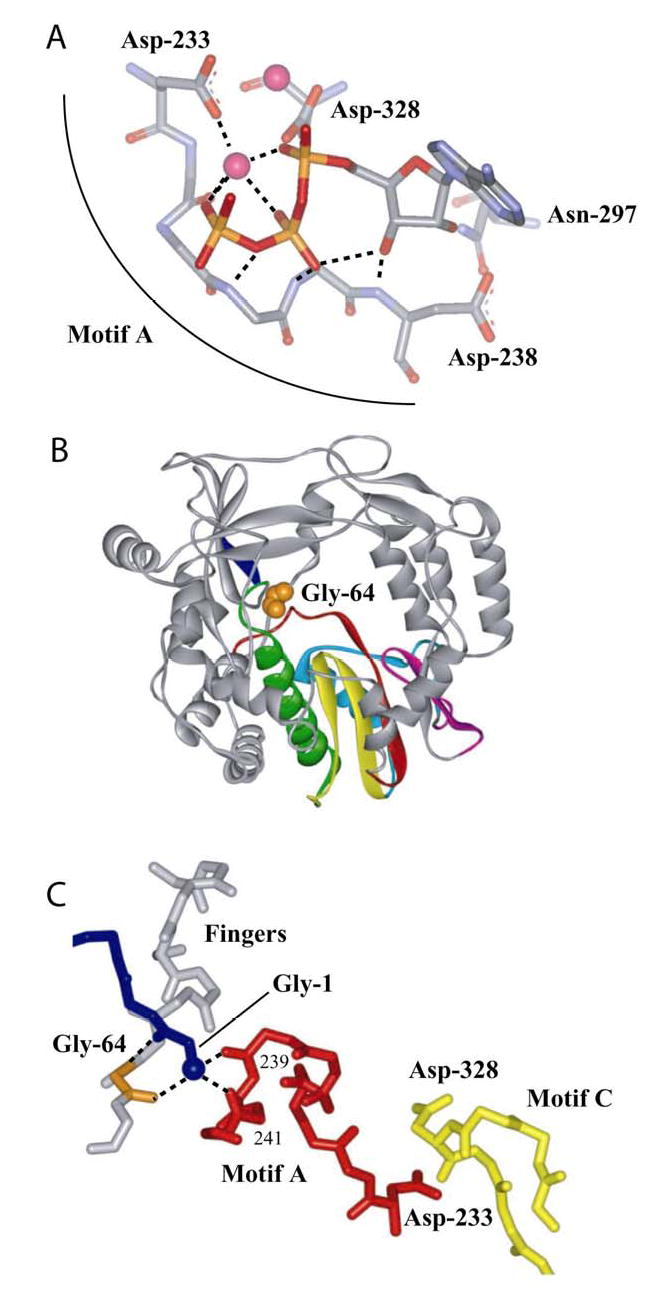
(A) Motif A functions, in part, to orient the triphosphate of the incoming nucleotide for catalysis. Model for interaction of 3Dpol with bound nucleotide (19,20). ATP and metal ions required for catalysis are labeled. In this model, the side chains for Asp-233 and Asp-238 have been rotated to permit interactions with ATP. (B) Gly-64 is located in the fingers subdomain. Complete structure for PV 3Dpol (22). The conserved structural motifs in the palm subdomain are colored as follows: motif A, red; motif B, green; motif C, yellow; motif D, blue; and motif E, purple. Van der Waal’s projection of Gly-64 (orange). (C) Gly-64 backbone orients the amino terminus, and the amino terminus interacts with motif A. The amino terminus of 3Dpol is in blue, Gly-64 is in orange, motif A is in red, motif C is in yellow and hydrogen bonds are shown as dashed lines. Misalignment of position 64 will cause defects to the orientation of the triphosphate as well as the efficiency of phosphoryl transfer owing to misalignment of motif A.
