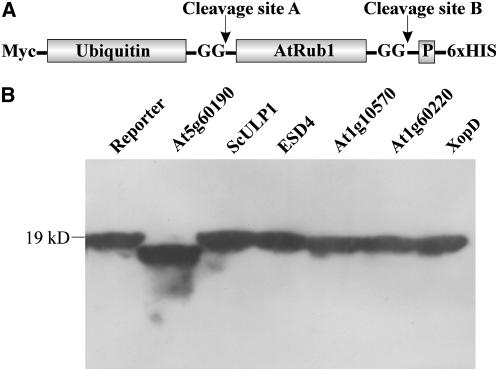
Figure 12.
Pre-Rub1 cleavage activity and substrate specificity of At1g60190. A, Structure of the reporter construct that was used to detect pre-AtRub1-processing activity. The reporter is based on a naturally occurring ubiquitin-pre-AtRub1 protein (At1g31340) that was fused with an N-terminal Myc tag and a C-terminal hexa-His tag. Two possible sites of protease cleavage are marked by arrows. The diglycine motifs that are expected to be exposed in the mature molecules are depicted, and the small prepeptide following AtRub1 is labeled with P. B, Pre-AtRub1-processing activity of At5g60190 (lane 2), ScULP1 (lane 3), ESD4 (lane 4), At1g10570 (lane 5), At1g60220 (lane 6), and XopD (lane 7) was determined. Proteins were separated on a 15% SDS-polyacrylamide gel, and cleavage of the reporter construct was detected by western-blot analysis using a Myc peptide-specific antiserum.