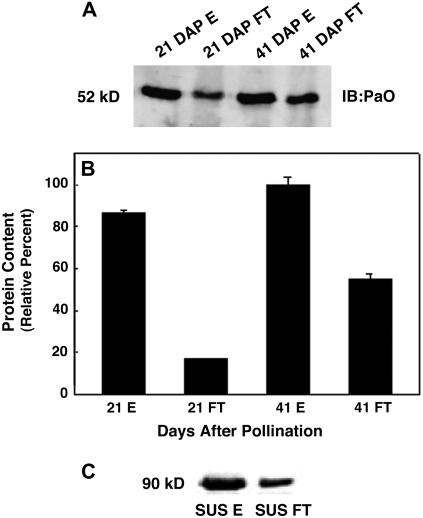
Figure 5.
IMAC revealed dynamic PaO phosphorylation during canola seed development. PaO phosphorylation was determined at 21 DAP when PaO protein was one-half of maximum but activity very low and at 41 DAP when both PaO protein and activity were highest. A PhosphoProtein Purification kit (Qiagen) was used to separate phosphorylated (elute [E]) and nonphosphorylated (flow through [FT]) canola seed membrane proteins. The PaO fractions were identified and quantified by immunoblot and infrared imaging. A, Solubilized total membrane fractions containing PaO were isolated in combination with protease and phosphatase inhibitors. Protein expression was measured based on isolated membrane fractions of canola seeds. B, PaO protein levels increased over the period of seed development. Quantification of the blot from A demonstrated that at 21 DAP, the eluted phosphorylated fraction (21 E) contained almost 5-fold higher amounts of PaO protein than the flow through nonphosphorylated fraction (21 FT). At 41 DAP, while phosphorylated PaO proteins (41 E) showed only a modest increase from 21 DAP, the nonphosphorylated fraction (41 FT) increased by >3-fold. PaO was detected with polyclonal antibodies from the maize LLS1 (PaO) protein. Equal amounts of protein from membrane fractions of canola seeds were loaded in each lane. The units for protein expression are intensity/pixel. C, Separation of phosphorylated (SUS E) and nonphosphorylated (SUS FT) maize leaf SUS.