Fig. 11.
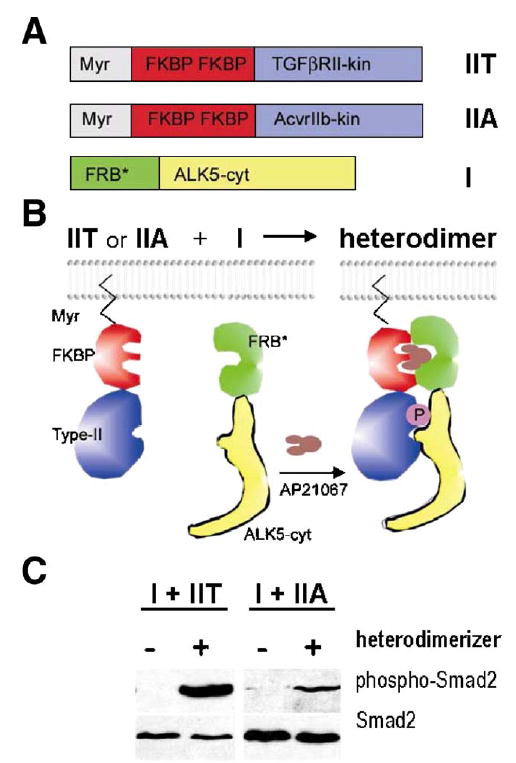
Activation of ALK5 by two different type II receptors in vitro. Regulated heterodimerization was used as a model system to test whether ALK5 may also be activated by ACVRIIB in addition to canonical TGFβRII. (A) Expression constructs for type II receptors contained N-terminal myristoylation signal (Myr), two copies of FK506 binding domains (FKBP), and regions coding for kinase domains of ACVRIIB (construct IIA in panel A) and TGFβRII (construct IIT in panel A), respectively. The ALK5 cytoplasmic domain was fused to FKBP-rapamycin binding domain (FRB*, see Materials and methods; construct I in panel A). (B) Heterodimerizing agent (AP21967, Ariad Pharmaceuticals; brown in the scheme) can be used to induce regulated heterodimerization of chimeric type I and type II receptor fusion proteins expressed in cell culture. (C) Western blot analysis shows that the kinase domain of ACVRIIB was able to activate the ALK5 fusion protein in Tgfbr2-deficient DR26 cells co-transfected with the Smad2 expression vector, albeit less efficiently than the corresponding TGFβRII domain, as demonstrated by phosphorylation of the effector protein Smad2. Left panel-exposure 5min; right panel—exposure 20min.
