Fig. 1.
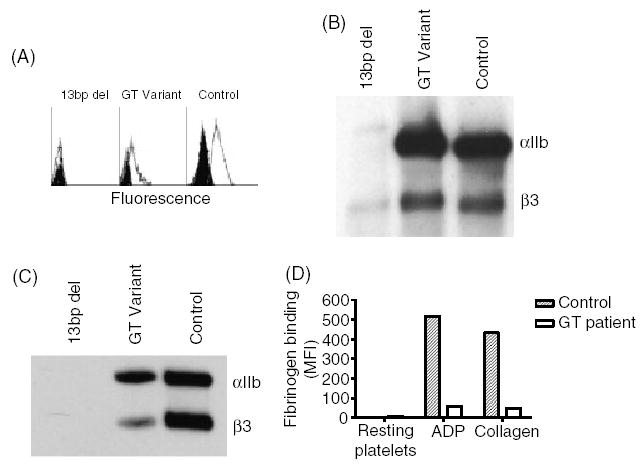
Platelet abnormalities in patients with Glanzmann thrombasthenia (GT). (A) Flow cytometric analysis of αIIbβ3 in platelets of a GT patient carrying the 13-bp deletion in the αIIb gene, a patient with a −3C → G mutation in intron 14–exon 15 junction of the β3 gene which causes variant GT, and a healthy control. fluorescein-isothiocyanate (FITC)-conjugated P2 monoclonal antibody against the αIIbβ3 complex was used. Background staining with FITC-conjugated mouse IgG is shown in black. (B) Immunoblotting under reduced conditions of sodium dodecyl sulphate-solubilized platelets using polyclonal antibodies against αIIb and β3. Notable is the trace amount of pro-αIIb and β3 in solubilized platelets of a patient with the 13-bp deletion and the normal amount of αIIb and β3 detectable in the patient with the variant mutation. (C) Immunoblot analysis under non-reducing conditions of αIIbβ3 immuno-precipitated by 10E5 monoclonal antibody, using SZ22 and AP3 monoclonal antibodies against αIIb and β3, respectively. Notable is the absence of αIIb and β3 in the patient with 13-bp deletion, and the reduced amounts of αIIb and β3 in the patient with the variant mutation compared with control. (D) Fibrinogen binding to resting platelets and platelets activated by 1.25 μm adenosine diphosphate or 1.25 μg mL−1 collagen. Note the impaired binding of fibrinogen to platelets of the patient with the variant mutation compared with control platelets.
