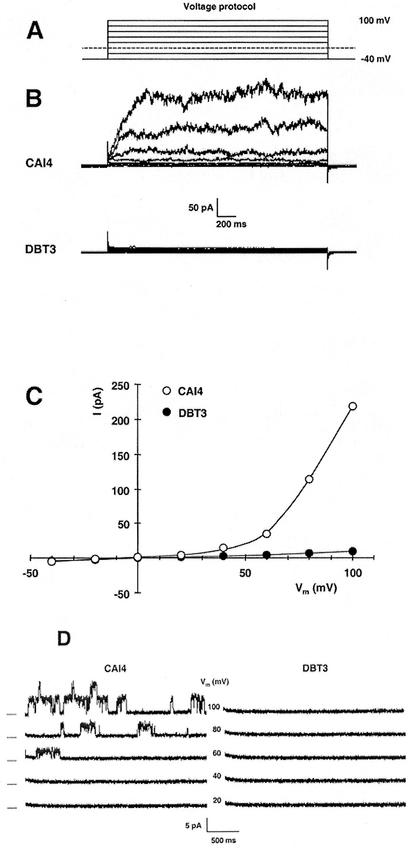
FIG. 4.
Disruption of the TOK1 gene in Candida removes outward rectifier currents that are characteristic of TOK1 proteins, as shown by patch-clamp records from C. albicans protoplasts. (A) Superimposed traces for the voltage-clamp protocol used in whole-cell recording. Membrane voltage was clamped, in 2.5-s pulses, from a holding potential of −40 mV to values of +100 mV to −40 mV in steps of −20 mV. (B) Superimposed current traces in response to the voltage-clamp protocol (panel A) for the wild-type strain CAI4 (TOK1/TOK1, upper record set) and for the fully disrupted strain DBT3 (tok1/tok1, lower record set). The outward currents, which were very similar to Tok1p-associated currents in Saccharomyces, were completely absent from DBT3. (C) Summary plot of the steady-state currents in panel B: for each pulse, current was averaged over the interval 1.2 to 2.2 s and plotted against the clamped membrane voltage. Values plotted are representative (near average) for 10 cells from each strain. (D) Current traces from an outside-out patch isolated from the wild-type strain (CAI4, left panel) and from the tok1-disrupted strain (DBT3, right panel). The voltage protocol was similar to that used for whole-cell recording, but with 3-s pulses. Dashes at the left indicate zero current for each pair of traces, and numbers in the middle are the clamped membrane voltages. The TOK1 patch contained at least two channels, each behaving similarly to Saccharomyces Tok1p, with a noisy open state previously shown to represent high-frequency close-open gating. Such single-channel events were completely absent from DBT3 patches. Data are representative of five patches for each strain.