Abstract
We studied the anti-Aspergillus activity of micafungin by using two fluorescent dyes to detect cell viability. Micafungin induced flattened hyphae, caused by the bursting of cells, which had lost their viability. Micafungin has killing activity against actively growing hyphae, even though it is not fungicidal against the whole burden of Aspergillus fumigatus.
Micafungin, an inhibitor of 1,3-β-d-glucan synthesis, shows incomplete growth inhibition of Aspergillus species in vitro (6, 13), and the MIC endpoint is defined as the minimum concentration resulting in prominent reduction in turbidity compared with growth control by using the broth microdilution method (11, 13). It has been reported, nevertheless, that micafungin has excellent activities in several mouse infection models of Aspergillus fumigatus (7, 10). It is considered that antifungal efficacy in animal models is the combined result of specific and/or nonspecific host defense systems, in addition to the direct antifungal effect of the drug itself. In order to characterize the antifungal activity, assessments of cell viability have been performed by using viability- and mortality-specific fluorescent dyes (3, 9). In an earlier report, Bowman et al. examined the dye-staining changes associated with caspofungin-induced loss of viability and concluded that caspofungin killed growing cells of A. fumigatus hyphae (3). In the present study, we confirmed the killing activity of micafungin, which has the same antifungal mechanism as caspofungin, against A. fumigatus by using the same fluorescent dye-staining method, in order to clarify the anti-Aspergillus character of micafungin.
Micafungin was synthesized at Fujisawa Pharmaceutical Co., Ltd. (Osaka, Japan) and was dissolved in distilled water. A. fumigatus TIMM0063 (MIC of micafungin, 0.0078 μg/ml) (13) was a kind gift from H. Yamaguchi, Teikyo University. For assessing cell viability, we used the same method as previously described (3). The supplemented RPMI 1640 medium was buffered with 165 mM 3-(N-morpholine) propane sulfonic acid (MOPS) (pH 7.0) containing 0.15% Junlon PW110 (Nihon Junyaku, Tokyo, Japan) to encourage dispersed hyphal growth of A. fumigatus (3, 8). The addition of Junlon to the RPMI medium did not affect the micafungin MIC for A. fumigatus TIMM0063 (data not shown). Briefly, a preculture of hyphal suspension of A. fumigatus was incubated with micafungin at 37°C for 7 h, and then the samples were stained with 5 (6)-carboxyfluorescein diacetate (CFDA, vitality dye; Sigma Chemical, St. Louis, Mo.) or bis-(1,3-dibutylbarbituric acid) trimethine oxonol [DiBAC4(3), mortality dye; Molecular Probes, Inc., Eugene, Oreg.]. Each sample was observed by using both differential interference contrast and fluorescent microscopy with Nomarski optics and a fluorescent detection system (AX-80, WIB cube; excitation, 460 to 490 nm; absorption, 515 nm; Olympus, Tokyo, Japan). The observations were performed with 2 slides per sample and 30 fields per slide. We confirmed good reproducibility by triple assays on different days. Micrographs that exhibited typical morphology are shown for each condition.
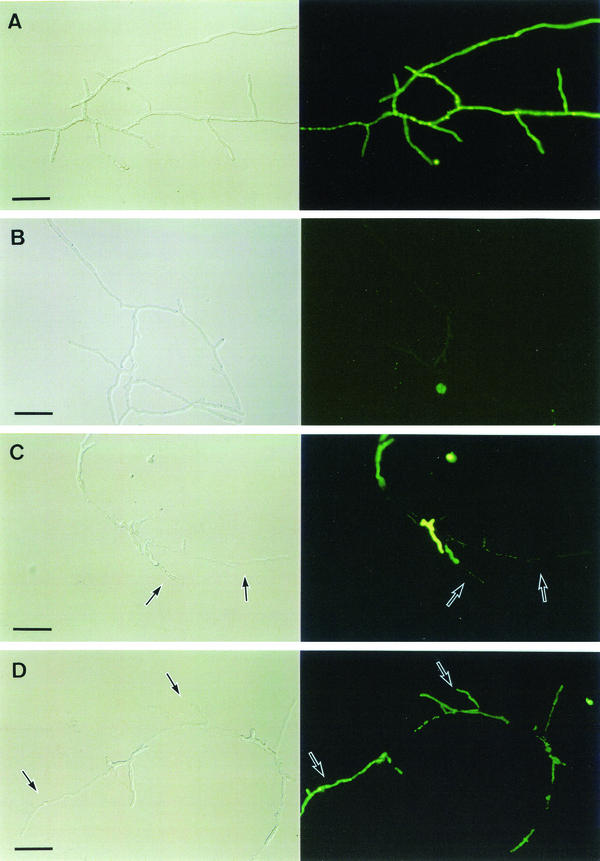
Hyphae of A. fumigatus were well extended with many branches after 7 h of incubation in the absence of micafungin, as assessed by differential interference contrast microscopic observation (Fig. 1A and B, left). CFDA is a lipophilic nonpolar substrate that penetrates into cytoplasm through the cell membrane and is hydrolyzed to the fluorescent carboxy fluorescein in live cells by intracellular esterases (4, 12). All hyphae were well stained with CFDA (Fig. 1A, right), which indicated that these cells possessed viability. DiBAC4(3) is an anionic lipophilic dye, highly sensitive to membrane potential. It enters cells via a depolarized plasma membrane, binds to phospholipids in the plasma membrane, and fluoresces (1, 5). Normal cells were scarcely stained with DiBAC4(3) (Fig. 1B, right), which indicated that these cells maintained viability due to the retention of membrane potential.
FIG. 1.
Discrimination of cell viability and mortality by using CFDA (A and C, right) and DiBAC4(3) (B and D, right) after exposure to 0.0078 μg of micafungin/ml (one times the MIC) (C and D) or to the controls (A and B) for 7 h. The left column shows Nomarski observations of the corresponding observations on the right. Arrows show flattened cells (left column images) and the corresponding cell images (right column images). Bars, 40 μm.
In contrast, after 7 h of exposure to 0.0078 μg of micafungin/ml (one times the MIC), hyphal cells at the hyphal tips or branch points appeared to be thin as a result of the emptying of cellular contents (Fig. 1C and D, left). These flattened cells were only slightly stained with CFDA (Fig. 1C, right), which indicated the loss of viability. These results were supported by the finding that flattened cells were markedly stained with DiBAC4(3) (Fig. 1D, right) due to the loss of membrane potential. These results indicated that micafungin induced hyphal cell killing.
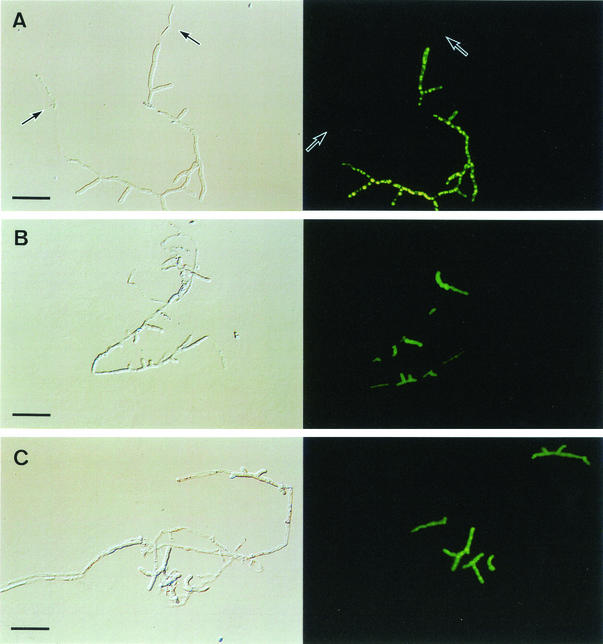
We examined the killing effect of micafungin at several concentrations against A. fumigatus hyphae. When exposed to four times the MIC of micafungin, killed cells were observed; however, a fraction of the cells still retained normal morphology, indicating their viability, similar to the result from one times the MIC (Fig. 2B and C). These results indicate that micafungin does not show complete killing against A. fumigatus at higher concentrations. Additionally, when exposed to one-fourth the MIC of micafungin, killed cells that had lost their viability were observed at the hyphal tips (Fig. 2A).
FIG. 2.
Killing activity of micafungin. Hyphae were exposed to one-fourth the MIC (A), one times the MIC (B), and four times the MIC (C) of micafungin for 7 h. The right columns were stained with CFDA, and the left columns are Nomarski observations of the corresponding observations from the right columns. Arrows show flattened cells (left column images) and the corresponding cell images (right column images). Bars, 40 μm.
In the earlier report, the authors demonstrated that caspofungin killed A. fumigatus at the hyphal tips and branch sites, and they also described the different patterns of killing caused by other antifungals. Amphotericin B killed whole cells, and itraconazole showed slight or partial killing (3). Micafungin is also a 1,3-β-d-glucan synthesis inhibitor like caspofungin and is therefore presumed to have the same killing activity. We confirmed that micafungin induced similar morphological changes and fluorescent findings to caspofungin by using the same method. These results support the proposal that this killing activity is characteristic of the mechanism of action.
In general, the fragile cell wall resulting from inhibition of 1,3-β-d-glucan synthesis is considered to be sensitive to outer osmotic pressure, which induces fungal cell killing (6). In the present study, micafungin showed killing activity against growing hyphae, but viable cells still remained. In addition, when micafungin was added to ungerminated conidia, hyphal elongation was inhibited by disruption of hyphal tips. As a result, shortened hyphae were observed, which were stained by CFDA (data not shown). These results are compatible with the fact that the MIC endpoint of micafungin for Aspergillus species is defined as a prominent reduction in turbidity by using the broth microdilution method. Furthermore, micafungin damaged cells of A. fumigatus at the hyphal tips or branch points, the actively growing portion of the cells where 1,3-β-d-glucan synthesis is located (2), and thus led to cell killing. It is suggested that this partial killing effect is one of the reasons that explains why micafungin shows potent anti-Aspergillus activity in vivo.
Acknowledgments
We thank David Barrett, Medicinal Chemistry Research Laboratories, Fujisawa Pharmaceutical Co., Ltd, for discussions and advice during the preparation of the manuscript.
REFERENCES
- 1.Bashford, C. L., B. Chance, J. C. Smith, and T. Yoshida. 1979. The behavior of oxonol dyes in phospholipid dispersions. Biophys. J. 25:63-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beauvais, A., J. M. Bruneau, P. C. Mol, M. J. Buitrago, R. Legrand, and J. P. Latgé. 2001. Glucan synthase complex of Aspergillus fumigatus. J. Bacteriol. 183:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman, J. C., P. S. Hicks, M. B. Krutz, H. Rosen, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2002. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob. Agents Chemother. 46:3001-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breeuwer, P., J. L. Dorcourt, N. Bunschoten, M. H. Zwietering, F. M. Rombouts, and T. Abee. 1995. Characterization of uptake and hydrolysis of fluorescein diacetate and carboxyfluorescein diacetate by intracellular esterases in Saccharomyces cerevisiae, which result in accumulation of fluorescent product. Appl. Environ. Microbiol. 61:1614-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epps, D. E., M. L. Wolfe, and V. Groppi. 1994. Characterization of the steady-state and dynamic fluorescence properties of the potential-sensitive dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3)) in model systems and cells. Chem. Phys. Lipids 69:137-150. [DOI] [PubMed] [Google Scholar]
- 6.Hatano, K., Y. Morishita, T. Nakai, and F. Ikeda. 2002. Antifungal mechanism of FK463 against Candida albicans and Aspergillus fumigatus. J. Antibiot. 55:219-222. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda, F., Y. Wakai, S. Matsumoto, K. Maki, E. Watabe, S. Tawara, T. Goto, Y. Watanabe, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis. Antimicrob. Agents Chemother. 44:614-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, P., D. Moore, and A. P. J. Trinci. 1988. Effects of Junlon and Hostacerin on the electrokinetic properties of spores of Aspergillus nigar, Phanerochaete chrysosporium and Geotrichum candidum. J. Gen. Microbiol. 134:235-240. [Google Scholar]
- 9.Liao, R. S., R. P. Rennie, and J. A. Talbot. 1999. Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob. Agents Chemother. 43:1034-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto, S., Y. Wakai, T. Nakai, K. Hatano, T. Ushitani, F. Ikeda, S. Tawara, T. Goto, F. Matsumoto, and S. Kuwahara. 2000. Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of pulmonary aspergillosis. Antimicrob. Agents Chemother. 44:619-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. NCCLS document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Pringle, J. R., R. A. Perston, A. M. Adams, T. Stearns, D. G. Drubin, B. K. Haarer, and E. W. Jones. 1989. Fluorescence microscopy methods for yeast. Methods Cell Biol. 31:357-435. [DOI] [PubMed] [Google Scholar]
- 13.Tawara, S., F. Ikeda, K. Maki, Y. Morishita, K. Otomo, N. Teratani, T. Goto, M. Tomishima, H. Ohki, A. Yamada, K. Kawabata, H. Takasugi, K. Sakane, H. Tanaka, F. Matsumoto, and S. Kuwahara. 2000. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob. Agents Chemother. 44:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]