Abstract
The activation of oxylipin-based chemical defense in the diatom Thalassiosira rotula is initiated by phospholipases that act immediately after cell damage. This lipase activity is responsible for the preferential release of free mono- and polyunsaturated fatty acids. Among these, eicosatetraenoic- and eicosapentaenoic acid are further converted by lipoxygenases to reactive defensive metabolites such as the antiproliferative α,β,γ,δ-unsaturated aldehydes 2,4-decadienal and 2,4,7-decatrienal. We show that mainly saturated free fatty acids are present in the intact diatom T. rotula, whereas the amount of free polyunsaturated eicosanoids is drastically increased in the first minutes after wounding. Using fluorescent probes, the main enzyme activity responsible for initiation of the aldehyde-generating lipase/lipoxygenase/hydroperoxide lyase cascade was characterized as a phospholipase A2. All enzymes involved in this specific defensive reaction are active in seawater over several minutes. Thus, the mechanism allows the unicellular algae to overcome restrictions arising out of potential dilution of defensive metabolites. Only upon predation are high local concentrations of aldehydes formed in the vicinity of the herbivores, whereas in times of low stress, cellular resources can be invested in the formation of eicosanoid-rich phospholipids. In contrast to higher plants, which use lipases acting on galactolipids to release C18 fatty acids for production of leaf-volatile aldehydes, diatoms rely on phospholipids and the transformation of C20 fatty acids to form 2,4-decadienal and 2,4,7-decatrienal as an activated defense.
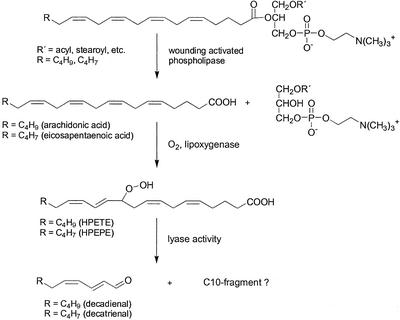
Seasonal blooms of phytoplankton are often dominated by diatoms, unicellular algae, that are regarded as most important primary producers sustaining the marine food chain. Given this central importance it is surprising that ecological studies of pelagic food chains were almost exclusively focused on the transfer of matter and energy between different trophic levels without paying attention to the individual species-specific defense. Even though in these studies diatoms are often considered as a high-quality food source, there have been observations of certain diatom species with a negative influence on the reproduction of herbivorous copepods (Ban et al., 1997). This effect has been attributed to the presence of inhibitory compounds in diatoms that reduce egg fecundity of herbivores (Ianora et al., 1996). Only recently, analysis of the antiproliferative components of extracts from the diatom Thalassiosira rotula resulted in the characterization of the reactive aldehydes (2E,4Z)-deca-2,4-dienal and (2E,4Z,7Z)-deca-2,4,7-trienal that inhibit egg cleavage of copepods (Miralto et al., 1999). The activity of these aldehydes can be attributed to a reactive Michael acceptor structure element (Vollenweider et al., 2000) that is widespread in different fatty acid-derived metabolites from diatoms (Pohnert and Boland, 1996; Jüttner and Durst, 1997; Pohnert, 2000). However, the concentrations of >0.5 mg of 2,4,7-decatrienal in 1 L of seawater, required for an inhibitory effect, are rather high for an efficient chemical defense in the dilute pelagic environment. In first biosynthetic investigations of these compounds, we could show that intact cells of T. rotula did not contain any of the inhibitory aldehydes and that aldehyde production is only activated after mechanical stress or cell disruption (Pohnert, 2000). The late biosynthetic steps toward these defensive metabolites most likely involve the action of a lipoxygenase/hydroperoxide lyase on polyunsaturated eicosanoic fatty acids (Scheme S1; Pohnert and Boland, 1996; Pohnert, 2000).
Scheme 1.
The proposed pathway for the wound-activated transformation of eicosanoic fatty acids in T. rotula.
However, nothing is known about the activation of this defense on demand. Further, only few studies on the activation of lipoxygenase-based aldehyde production exist for higher plants. Matsui et al. (2000) identified a not further-characterized lipid-hydro-lyzing activity acting on galactolipids involved in the formation of the green leaf-volatile hexanal in Arabidopsis. Other lipid-hydrolyzing activities identified from higher plants include phospholipases that are involved in the regulation of the jasmonic acid branch of the oxylipin pathway. These lipases can be induced by plant-defensive elicitors (Chandra et al., 1996; Chapman, 1998; Wang, 2001). But despite some work on the quantification of free and lipid bound fatty acids in diatoms that suggests the action of lipases upon cell lysis, the regulation of the release of specific fatty acids by these algae is not verified (Berge et al., 1995; Budge and Parrish, 1999).
The present study shows that cells of the diatom T. rotula are able to invest their metabolic energy in times of low stress into the formation of lipids such as phospholipids that are rapidly and efficiently cleaved by phospholipases upon cell damage. The released eicosanoic fatty acids then immediately serve as precursors for defensive metabolites.
RESULTS
Substrates for the Lipoxygenase/Hydroperoxide Lyase from T. rotula
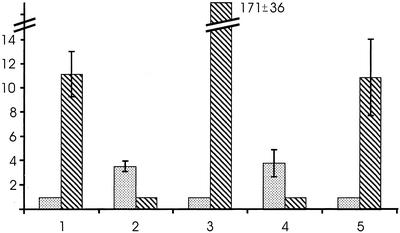
Solid-phase microextraction (SPME) of volatiles from damaged cells of T. rotula show up to 0.72 pg cell−1 of (2E,4Z)-deca-2,4-dienal and (2E,4Z,7Z)-deca-2,4,7-trienal (Pohnert, 2000). If cells were harvested in the early stationary phase of growth and wounded by sonication, the ratio of these aldehydes was determined to 1:11.1 ± 1.9 (ratio of gas chromatographic [GC]-integrals) 2,4-decadienal:2,4,7-decatrienal (Fig. 1). External addition of free [2H8]-arachidonic acid after wounding led to a pronounced increase of labeled 2,4-decadienal to a ratio of 1:0.28 ± 0.1 (GC-integrals) 2,4-decadienal:2,4,7-decatrienal with >80% (determined by mass spectrometry) labeled 2,4-decadienal (Fig. 1). Analysis of the labeling pattern showed clearly that 2,4-decadienal is formed from the C11-C20 terminus of the administered arachidonic acid. Addition of eicosapentaenoic acid (C20:5 ω3) before cell disruption resulted in the preferential production of 2,4,7-decatrienal with a higher degree of unsaturation (1:171 ± 36 [GC-integrals] 2,4-decadienal:2,4,7-decatrienal; Fig. 1). In contrast, addition of shorter chain fatty acids like linolenic- or octadecatetraenoic acid (C18:4 ω3), which have a terminus corresponding to the aldehyde geometry, did not result in any change of the 2,4-decadienal to 2,4,7-decatrienal ratio. The substrate specificity for C20 fatty acids is further confirmed by the fact that the addition of a longer chain acid (C22:6 ω3), also present in T. rotula, did not show any effects on the 2,4-decadienal to 2,4,7-decatrienal ratio. This indicates that exclusively C20 fatty acids serve as substrates for the production of the volatile aldehydes. To challenge substrate tolerance of the involved enzymes, [2H8]methyl arachidonate was added before disruption of the cells. The presence of this derivative did not result in significant production of labeled 2,4-decadienal, showing the requirement of free fatty acids as lipoxygenase substrates. The detected traces of labeled 2,4-decadienal might be attributable to the presence of acid-releasing esterases, previously detected after lysis of diatoms (Minier et al., 1993; Agusti and Duarte, 2000). The fact that addition of [1,2,3]-tri[arachidonyl]glycerol did not effect the aldehyde ratio (see below) also confirms the substrate preference for free fatty acids. This preference for free fatty acids is also known for most lipoxygenases from higher plants, albeit here C18 fatty acids exclusively serve as substrates for the formation of the shorter chain nonconjugated aldehydes 3Z-hex-3-enal, hexanal, 3Z-non-3-enal, and 3Z,6Z-nona-3,6-dienal (Gardner, 1991; Blee, 1998).
Figure 1.
Relative ratio of 2,4-decadienal (dotted) and 2,4,7-decatrienal (hatched) in wounded T. rotula (n = 3). 1, Ten minutes after sonication; 2, same as 1 with externally added [2H8]arachidonic acid; 3, same as 1 with externally added eicosapentaenoic acid; 4, same as 1 with previous addition of 1-octadecanoyl-2-arachidonyl-sn-glycero-3-phosphocholine; 5, same as 1 with previous addition of [1,2,3]-tri[arachidonyl]glycerol.
Quantification of Free Fatty Acids in T. rotula
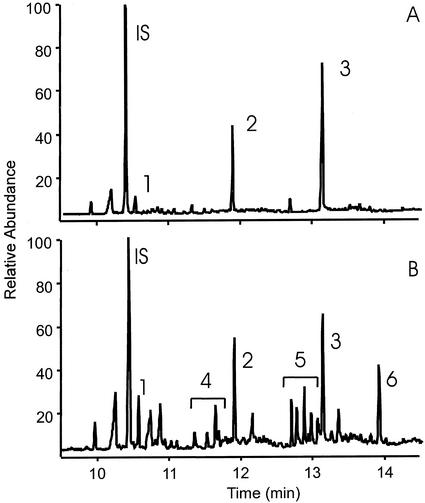
To determine whether the lack of defensive metabolites in intact T. rotula cells was due to a lack of free fatty acids, the amount of these substrates was determined in intact cells. This was achieved after careful isolation of the cells using centrifugation at low speed and prevention of lipase reactions during work-up by addition of boiling water before extraction (Berge et al., 1995; Budge and Parrish, 1999). The free fatty acids were derivatized and determined as methyl esters using gas chromatography (GC)-mass spectrometry (MS). Only the saturated fatty acids C14:0 (0.2 ± 0.08 pg cell−1), C16:0 (0.8 ± 0.28 pg cell−1), and C18:0 (2.2 ± 0.8 pg cell−1) were found in significant amounts (Fig. 2A). Remarkably, these measurements showed a nearly complete lack of free mono- and polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid, the precursor of 2,4,7-decatrienal. This situation changes drastically if the extraction of free fatty acids is performed 10 min after cell disruption through sonication. Here, an increase of the overall amount of free fatty acids that is most strongly seen for PUFAs is observed (Fig. 2B). The most pronounced increase was observed for eicosapentaenoic acid, with levels of up to 1.6 ± 0.8 pg cell−1. However, the level of unsaturated C18 and C16 fatty acids was also increased relative to the boiled control. In these experiments, no efforts were taken to inhibit the metabolism of eicosapentaenoic acid to 2,4,7-decatrienal and other oxylipins; thus, the absolute amount of released eicosapentaenoic acid will be even higher.
Figure 2.
GC separation of free fatty acids (as methyl esters) in T. rotula. A, Lipase reactions were suppressed by adding boiling water to the carefully isolated intact cells before extraction. B, Cells were disrupted by sonication and extracted after 10 min. IS, Internal standard; 1, myristic acid (C14:0); 2, palmitic acid (C16:0); 3, stearic acid (C18:0); 4, unsaturated C16:n fatty acids; 5, unsaturated C18:n fatty acids; 6, eicosapentaenoic acid (C20:5).
Inhibition of the Lipase Activity in T. rotula Cells after Damage
Inhibitor experiments showed that the release of free fatty acids by lipolytic enzymes is the deciding step in the activation of the defensive reaction of T. rotula. If lipase activities were inhibited with quinacrine, an inhibitor of animal phospholipase A2 (Strosznajder and Samochocki, 1991; Schiess et al., 1992), the overall amount of aldehydes released was reduced concentration dependent. Quinacrine (20 μm), added to the medium before sonication of the diatoms, inhibited the release of 2,4-decadienal and 2,4,7-decatrienal to about 60% (Table I).
Table I.
Aldehyde production after inhibition of lipase activity
| Treatment | Decadienal and Decatrienal |
|---|---|
| fmol cell−1 | |
| Control | 4.2 ± 0.8 |
| 20 μm Quinacrine | 2.5 ± 0.9 |
| 100 μm Quinacrine | <0.5 |
| 100 μm Quinacrine + [2H8] − C20:4 | 8.2 ± 2.7a |
Degree of labeled [2H4]-decadienal > 90%.
Addition of 100 μm quinacrine to the medium before cell damage totally suppressed the aldehyde-production. Addition of free [2H8]-arachidonic acid to 100 μm quinacrine-inhibited cell suspensions resulted in increased levels of [2H4]-decadienal, comparable with those obtained by addition of the labeled acid to uninhibited cells. This shows that the inhibition was due to reduced levels of free fatty acids after quinacrine treatment and that the inhibitor did not interfere with the lipoxygenase/lyase reaction itself (Table I).
Specificity of Lipases from T. rotula
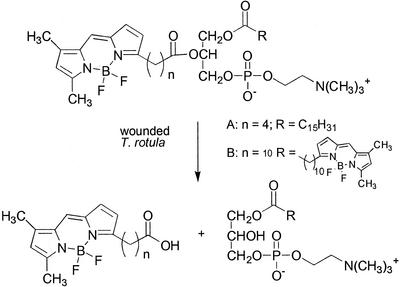
To assess the type of lipase activity involved in the formation of free fatty acids during tissue disruption, the transformation of different lipid substrates was examined. Because the animal phospholipase inhibitor quinacrine affected the release of the defensive aldehydes, the initial focus was set on phospholipase activities found in T. rotula. Product analysis after administration of 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene (BODIPY)-labeled phospholipids allows the determination of the nature of the lipase activity involved. The resulting lysolipids are distinguishable by their chromatographic properties and characteristic fluorescence when excited with UV light (488 nm; Scheme S2).
Scheme 2.
The transformation of fluorescent-labeled phospholipids by T. rotula. Scheme 2A shows 2-(BODIPY-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine, and Scheme 2B shows 1,2-bis-(BODIPY-3-undecanoyl)-sn-glycero-3-phosphocholine.
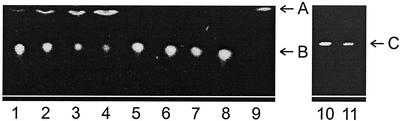
If labeled 2-(BODIPY-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine is added to wounded T. rotula cells in seawater, a preferential cleavage of the phospholipid in sn2 position is observed (Fig. 3, left). No other dominant fluorescent products from phospholipases with different site specificity were detected. This phospholipase is not released in significant amounts into the water as long as the cells are intact (Fig. 3, lines 5–7). Directly after cell disruption, however, the lipases are active and the onset of BODIPY-pentanoic acid release from the sn2 position can already be observed in the 1st min after damage. Because the wound-activated defensive reaction of the unicellular algae has to be active in seawater after cell disruption, one major prerequisite for an efficient defensive mechanism is the stability of the activated enzymes involved. Figure 3 shows that this is fulfilled because PLA2 is active over at least 20 min.
Figure 3.
Left, Activity of phospholipase A2 (PLA2) in seawater medium after cell disruption. Thin-layer chromatography (TLC) separation of phospholipids and lysolipids after administration to damaged and intact T. rotula (silica; MeOH/CHCl3/HOAc; A, BODIPY-3-pentanoic acid; B, 2-(BODIPY-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine). Lines 1 through 4, Labeled phospholipid treated with broken cells of T. rotula after 1, 3, 8, and 20 min; line 5 through 7, control treatments of intact cells after 1, 8, and 20 min show no PLA2 activity in the medium; line 8, labeled phospholipid; line 9, labeled phospholipid treated with bee venom (Sigma, Deisenhofen, Germany) phospholipase A2. Right, Test for triacylglycerol lipase activity [silica; ether; C, glycerol tris-(1-pyrenebutyrate)]. Line 10, Labeled tris-acylglycerol treated for 20 min with broken cells of T. rotula, no lysolipids, and labeled fatty acids with lower ratio of fronts compared with glycerol tris-(1-pyrenebutyrate) are detected; line 11, labeled lipid.
To test whether nonpolar lipids such as triacylglycerols, often detected in diatoms (Dunstan et al., 1994), might also serve as a source of free fatty acids, the fluorescent-labeled [1,2,3]-tri[1-pyrenebutyryl]glycerol was administered to damaged T. rotula. TLC analysis revealed that this substrate is not accepted by T. rotula lipases because no labeled cleavage products attributable to a triacylglycerol-hydrolyzing activity were detected within 20 min after wounding (Fig. 3, right).
Activity of the Involved PLA2
The question arose if the observed high PLA2 activity could really account for the sufficient release of aldehyde precursors after wounding. This could be addressed exploiting the fact that the 2,4,7-decatrienal to 2,4-decadienal ratio in wounded T. rotula is directly correlated with the presence of substrate fatty acids in the medium (Fig. 1). This allows one to test if addition of lipids rich in arachidonic acid results in increased 2,4-decadienal to 2,4,7-decatrienal ratios as it was observed after addition of free arachidonic acid. Addition of 1-octadecanoyl-2-arachidonyl-sn-glycero-3-phosphocholine to the medium before initiation of the defense cascade led to a significant increase of 2,4-decadienal compared with the untreated cells. After 10 min, the newly formed 2,4-decadienal reached the same level as observed after addition of free arachidonic acid (Fig. 1). The identified lipid-hydrolyzing activity thus can account for the amount of reactive aldehydes detected after cell disruption. In accordance with the results from TLC analysis after addition of labeled fluorescent triacylglyceride (Fig. 3), addition of [1,2,3]-tri[arach-idonyl]glycerol prior to cell disruption did not effect the 2,4-decadienal to 2,4,7-decatrienal ratio (Fig. 1).
Localization of the Phospholipase Activity
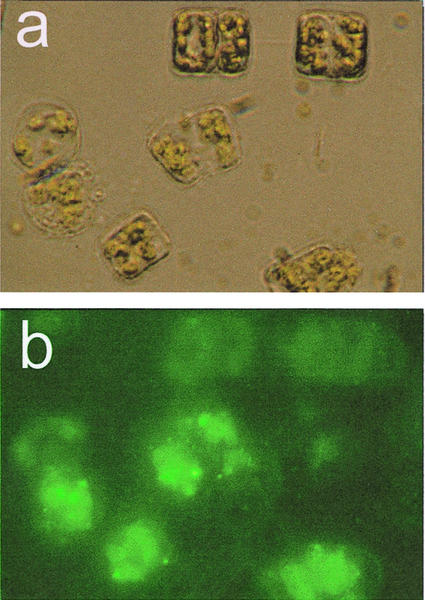
TLC analysis showed that the PLA2 from T. rotula has a high substrate tolerance, accepting even the double-labeled 1,2-bis-(BODIPY-3-undecanoyl)-sn-glycero-3-phosphocholine. This allowed the visualization of the lipase activity in damaged cells. Whereas the 1,2-bis-BODIPY-labeled phospholipid shows little fluorescence due to self-quenching of the two neighboring BODIPY residues, the resulting lysolipid, as well as the released free fatty acid, become fluorescent after the phospholipase A action (Paul et al., 1998; Roos et al., 1999). Application of this probe to intact cells of T. rotula before preparation followed by observation under an epifluorescence microscope showed clearly increased PLA2 activity around the extruding cytoplasm of damaged cells. In contrast, intact cells did not display any increased fluorescence attributable to external lipases (Fig. 4).
Figure 4.
Epifluorescense microscopy of intact and disrupted T. rotula treated with the fluorogenic substrate 1,2-bis-(BODIPY-3-undecanoyl)-sn-glycero-3-phosphocholine. A, Light microscopic control of the cells (left, intact cell; right, cell damaged during preparation). B, Same part, excited with UV light: λex = 450 to 490 nm and λem = 515 to 565 nm. The green fluorescence is due to the presence of lysolipids after action of T. rotula PLA2.
DISCUSSION
To understand the initiation of the wound-activated chemical defense in T. rotula, a series of experiments was conducted elucidating the precursors of the antiproliferative aldehydes and the regulation of their production. We established that exclusively free C20 fatty acids serve as substrates for the production of 2,4-decadienal and 2,4,7-decatrienal by T. rotula. This substrate specificity is also observed for the lipoxygenase-based production of volatile hydrocarbons by other diatoms. For example, the fresh water diatom Gomphonema parvulum produces the C11 hydrocarbon hormosirene, together with the highly reactive (5Z,7E)-9-oxo-nona-5,7-dienoic acid from eicosapentaenoic acid, but does not accept shorter chain fatty acids as substrates (Pohnert and Boland, 1996). In contrast, higher plants use exclusively linolenic and linoleic acid (C18) for the production of green leaf volatiles such as nonadienal, hexanal, and hexenal (Blee, 1998). Extractions after external application of C20 fatty acids show that the limiting factor for 2,4-decadienal- and 2,4,7-decatrienal-production is the supply of free fatty acids, and that the production of these aldehydes can be significantly increased by supplying the eicosanoid precursors. The incorporation of both eicosapentaenoic acid (C20:5 ω3) and arachidonic acid (C20:4 ω6) into the respective aldehydes 2,4,7-decatrienal and 2,4-decadienal shows that the involved lipoxygenase/hydroperoxide lyase from T. rotula has some substrate tolerance for the terminus of the C20 fatty acids. In contrast, it discriminates strongly in favor of a free fatty acid head group because neither methyl esters nor triacylglycerols are transformed. This preference for free fatty acids as substrates has also been observed for most lipoxygenases of higher plants, although some lipoxygenases acting on the lipid body have been reported (Hause et al., 2000; Feussner et al., 2001).
Because only saturated free fatty acids were found after careful isolation of intact T. rotula cells, and no direct substrates for the T. rotula lipoxygenases were present (Fig. 2A), it can be concluded that the availability of free fatty acids is the limiting factor for the aldehyde production. The preferred fatty acids released after wounding are mono- and PUFAs (Fig. 2B). This selectivity in the release of certain classes of fatty acids has been found for the first time in diatoms. The few studies on the lipid profile of intact or stressed diatoms focused on the overall lipid composition (Berge et al., 1995; Budge and Parrish, 1999). Whereas in intact Sceletonema costatum, no free fatty acids were present at all (Berge et al., 1995), a comparison of lipid and fatty acid composition of intact isolated and disrupted Pseudo-nitzschia sp. showed only an overall increase of free fatty acids after wounding of the cells (Budge and Parrish, 1999). Apparently, T. rotula and the investigated Pseudo-nitzschia sp. are able to store free fatty acids in their tissue, compounds that are only detected in trace amounts in most other living organisms. Moreover, T. rotula has developed a way to specifically increase the level of PUFAs upon cell disruption, thus providing the precursors for its defensive reaction against herbivores. The preferred fatty acids released after wounding are mono- and PUFAs (Fig. 2B). This selectivity in the release of unsaturated free fatty acids has just recently also been found for damaged fresh water biofilms containing predominantly diatoms. The free fatty acids act there as direct chemical defense against the grazer Thamnocephalus platyurus (Jüttner, 2001). In contrast, the regulation of the production of saturated aldehydes such as tridecanal from myristic acid, found in high quantities after wounding (G. Pohnert, unpublished data), must follow a different mechanism. Here, another lipase-independent reaction, like, e.g. a wound-activated action of an α-oxygenase (Kajiwara et al., 1994; Akakabe et al., 1999), could be operative because the substrates are already present in the intact cells.
The experiments with fluorescent markers and the inhibitor quinacrine show that phospholipids are accepted as preferred substrates for the release of C20 fatty acids. Use of 2-(BODIPY-3-pentanoyl)- 1-hexadecanoyl-sn-glycero-3-phosphocholine allows the determination of the site specificity of the phospholipases involved. The fluorescent products after action of different phospholipases are distinguishable by their ratio of front values in TLC analysis. As can be seen in Figure 3, only BODIPY-1-pentanoic acid was detected in addition to the fluorescent substrate after incubation with a sonicated suspension of T. rotula cells. No other fluorescent lysolipids were present, confirming the dominant action of a sitespecific PLA2. The positional distribution of fatty acids in lipids from diatoms has been investigated previously, using Phaeodactylum tricornutum as a model. In that study, eicosapentaenoic acid was found in both sn-1 and sn-2 positions of different phospholipids, as were other unsaturated C16 and C18 fatty acids (Arao et al., 1987; Yongmanitchai and Ward, 1993). Cleavage of phospholipids in the sn-2 position thus could result in the preferential release of mono- and PUFAs, as observed here. Using fluorescent substrates, we could show that the T. rotula PLA2 is active over at least 20 min in seawater (Fig. 3), thus providing a constant source for free fatty acids to be transformed to 2,4-decadienal and 2,4,7-decatrienal during the feeding process, e.g. of herbivorous copepods. It has been shown in previous work that the lipoxygenase/hydroperoxide lyase involved in aldehyde formation also retains activity in seawater over several minutes (Pohnert, 2000) so that the whole sequence of activated defense is operative under environmental conditions during the feeding process.
In contrast to the polar phospholipids, triacylglycerols, reported to be present in diatoms in significant amounts (Dunstan et al., 1994; Brown et al., 1996), did not serve as precursors for the aldehyde production (Fig. 1). The experiments with labeled phospholipids and triacylglycerols do not rule out the possibility of other lipid hydrolyzing activities to be involved in the activated defense of T. rotula. Because the presence of other polar lipids rich in eicosapentaenoic acid, such as monogalactosyldiacylglycerol, digalactosyldiacylglycerol, or sulfoquinoyosyldiacylglycerol have been reported from diatoms (Arao et al., 1987; Yongmanitchai and Ward, 1993), these sources have also been taken into account. Nevertheless, our experiments show that the involved phospholipase(s) are able to provide enough arachidonic acid for an increased 2,4-decadienal production if the arachidonic acid-rich phospholipid 1-octadecanoyl-2-arachidonyl-sn-glycero-3-phosphocholine is added (Fig. 1). The level of 2,4-decadienal observed after addition of this phospholipid was comparable with that after addition of free arachidonic acid. Lipases acting on added phospholipids thus released enough substrate to supply downstream lipoxygenases with substrates for 2,4-decadienal production. This confirms that PLA2 is the main lipase involved in the defensive reaction. The mode of activation of the defensive reaction in diatoms differs from that in higher plants. It has been reported just recently that a lipid-hydrolyzing activity acting on galactolipids is involved in the formation of hexanal upon disruption of Arabidopsis leaves (Matsui et al., 2000). This first study on the activation of aldehyde production in higher plants describes an uncharacterized lipase activity releasing linolenic acid that is subsequently transformed to hexanal.
Epifluorescence microscopy shows that T. rotula phospholipases present after cell disruption are most active around the effluent cytoplasm (Fig. 4). Apparently, a compartmentation separating PLA2 from the phospholipids prevents the release of PUFAs from polar lipids in intact cells. Cell disruption then brings the lipids into contact with the lipase and, presumably, with the lipoxygenase/hydroperoxide lyase responsible for aldehyde production. The question of whether, in addition, cofactors like Ca2+ ions are involved in the activation of the PLA2 will have to be answered by further biochemical characterization of the enzyme. These upcoming studies will also have to address in more detail the type of PLA2.
This mechanism of wound-activated chemical defense could provide a way to obtain high local concentrations of the defensive metabolites exclusively upon predation because the production of the aldehydes will occur in close vicinity of the feeding organs of copepods and might even be active after ingestion in the near neutral environment of the copepod gut. From an evolutionary viewpoint, this defensive reaction seems to make little sense for an individual unicellular diatom that will not be able to survive after cell damage. However, there will be a benefit to the population of genetically closely related or identical diatoms present in the water column during a bloom period of the diatoms because the pool of grazers will be reduced (Wolfe, 2000). This way to maintain an efficient chemical defense only on demand can reduce the risk of self-poisoning of the unicellular algae by the aggressive aldehydes. Moreover, the activated defense strategy described here allows T. rotula to invest the cellular resources into the production of storage lipids in times of low herbivore pressure.
MATERIALS AND METHODS
Culture
The culture of Thalassiosira rotula was obtained from Dr. Serge Poulet (Station Biologique, Roscoff, France). It originated from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton (West Boothbay Harbor, ME) strain CCMP 1647. T. rotula was grown in standing cultures at 17°C in artificial medium (Maier and Calenberg, 1994) during a 9- to 14-d growth period to a final concentration of about 2.5 × 105 cells mL−1. Illumination was provided on a 14:10 light:dark cycle (light intensity: 117 μE m−2 s−1).
Fatty Acid Analysis
Analysis of free fatty acids was performed following a modified procedure of Budge and Parrish (1999). Cells were harvested in their early stationary phase and carefully concentrated by centrifugation at low speed (1,000g). The pellets (40–100 mg wet weight) were either rediluted with water or growth medium to 10 mL or treated with boiling water (10 mL) to suppress lipase activity. For investigation of wounded cells, the non-boiled samples were sonicated using four 80-W, 5-s pulses of a 1000L Sonicator (B. Braun Biotech, Melsungen, Germany; 40%–60% cell disruption, judged by light microscopy) and extracted after 10 min. Boiled controls were extracted after recooling in an ice bath. For extraction, 37 mL of CHCl3 and MeOH (1:2 [w/v]) was added. One hundred micrograms of [2H27]-tetradecanoic acid was added as a standard. After shaking for 5 min, 17 mL of CHCl3 and 17 mL of water were added. The organic phase was collected and dried using MgSO4. After esterification with diazomethane, fatty acids were analyzed by GC-MS (Finnigan Trace MS [Thermo Finnigan, San Jose, CA], equipped with a 30-m Alltech [Deerfield, IL] DB225 MS column [id = 0.25 mm, 25-μm film thickness, and temperature program: 60°C {2 min} ramped at 5°C min−1 to 300°C {2 min}]).
Analysis of Volatile Aldehydes
For analysis of volatiles, 50 mL of a culture in the early stationary growth phase (about 107 cells) were concentrated and rediluted to 3 mL, transferred to 5-mL glass vials, and sonicated as described above. The samples were directly sealed after sonication using a Teflon cap. A polydimethylsiloxane-coated (100 μm) SPME fiber (Supelco, Bellefonte, PA) was introduced in the headspace over the medium. Extraction was performed for 5 or 10 min at room temperature. Evaporation of the volatiles from the fiber was directly performed within the injection port (260°C) of the GC-MS (DB225-MS, T program: 50°C [2 min, splitless] ramped with 10°C min−1 to 200°C and then with 30°C min−1 to 280°C). Unsaturated aldehydes were identified as described (Pohnert, 2000).
Lipid and Fatty Acid Transformation by T. rotula
Before experiments with lipid or fatty acid substrates, stock solutions (10 mg mL−1 in ethanol) were tested for the presence of aldehydes from auto-oxidation. If required, the commercially available substrates (Sigma, Deisenhofen, Germany; Cayman Chemicals, Ann Arbor, MI) were purified by chromatography. One hundred micrograms of the different substrates (Fig. 1) in ethanol was added to concentrated cultures (about 107 cells/3 mL) prepared as described for the extraction of aldehydes. For 10 min after sonication, SPME was performed as described above.
Inhibitor Treatments
Cells were concentrated by centrifugation as described above and resuspended carefully with 3 mL of the medium containing quinacrine added previously from a 10 mm stock solution in ethanol. The cell suspensions were pre-incubated for 15 min at room temperature before sonication and addition of undecane as a standard. The volatile aldehydes were extracted for 10 min as described above. To test for lipoxygenase/hydroperoxide lyase activity, a second set of samples, pretreated with 100 μm quinacrine, were supplied with 100 μg of [2H8]-arachidonic acid as a 10 mg mL−1 solution in ethanol before sonication and extraction. All experiments were replicated in triplicate.
Monitoring of PLA Activity
For TLC of lipase-reaction products, cells were harvested and concentrated as described above. Twenty microliters of the substrate 2-(BODIPY-3-pentanoyl)-1-hexadecanoyl-sn-glycero-3-phosphocholine (Molecular Probes, Leiden, The Netherlands) was added from a 1 mg mL−1 stock solution in ethanol to about 107 cells in 5 mL of seawater. The set-up was sonicated as described above and subsequently shaken continuously. Two hundred-microliter samples were taken every minute and directly extracted with 200 μL of CHCl3/0.5% (w/v) acetic acid. Ten microliters of the organic phase was loaded on a TLC plate (silica gel 60 F254, Merck, Darmstadt, Germany) and separated with CHCl3:MeOH:AcOH (65:25:10 [w/v]) as mobile phase. The fluorescent substrate and the lipase products were visualized under a UV lamp and their ratio of fronts compared with products from phospholipase reactions with commercially available phospholipases. As control, the above treatments were performed on intact cells without previous sonication.
Test for Triacylglycerol Lipase Activity
To test for triacylglycerol lipase activity, glycerol tris-(1-pyrenebutyrate) (Molecular Probes) was used. Due to low solubility, the substrate was added as a solution of 100 μg mL−1 dimethyl sulfoxide before sonication. Extraction was performed as described above and TLC separations with ether as mobile phase were visualized by UV light.
Epifluorescence Microscopy
Cells were harvested as described above. The fluorogenic substrate 1,2-bis-(BODIPY-3-undecanoyl)-sn-glycero-3-phosphocholine (Molecular Probes) was added in excess from a 1 mg mL−1 stock solution in ethanol. For microscopic measurements, about 50 μL of the cell suspension was treated with the marker on an object slide and protected with a coverslip. The documentation was performed 20 min thereafter. Cells damaged during the isolation procedure were distinguishable from intact ones by their fluorescence properties if filters were selected for λex (excitation wavelength) = 450 to 490 nm and λem (emission wavelength) = 515 to 565 nm. Fluorescent images were captured and digitalized with a Super Coolscan 4000 (Nikon, Tokyo).
ACKNOWLEDGMENTS
I gratefully acknowledge Dr. S. Poulet (Roscoff, France) for his advice and the gift of T. rotula. I am thankful to Prof. Wilhelm Boland (MPI, Jena, Germany) and Dr. Iro Feussner (IPB, Gatersleben, Germany), Verena Jung and Dr. Jörn Piel (both MPI) for stimulating discussions during the preparation of the manuscript. Andrea Lehr (MPI) is acknowledged for culturing of diatoms.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010974.
LITERATURE CITED
- Agusti S, Duarte CM. Strong seasonality in phytoplankton cell lysis in the nw Mediterranean littoral. Limnol Oceanogr. 2000;45:940–947. [Google Scholar]
- Akakabe Y, Matsui K, Kajiwara T. Enantioselective alpha-hydroperoxylation of long-chain fatty acids with crude enzyme of marine green alga Ulva pertusa. Tetrahedron Lett. 1999;40:1137–1140. [Google Scholar]
- Arao T, Kawaguchi A, Yamada M. Positional distribution of fatty-acids in lipids of the marine diatom Phaeodactylum tricornutum. Phytochemistry. 1987;26:2573–2576. [Google Scholar]
- Ban SH, Burns C, Castel J, Chaudron Y, Christou E, Escribano R, Umani SF, Gasparini S, Ruiz FG, Hoffmeyer M et al. The paradox of diatom-copepod interactions. Mar Ecol Prog Ser. 1997;157:287–293. [Google Scholar]
- Berge JP, Gouygou JP, Dubacq JP, Durand P. Reassessment of lipid-composition of the diatom Skeletonema costatum. Phytochemistry. 1995;39:1017–1021. [Google Scholar]
- Blee E. Phytooxylipins and plant defense reactions. Prog Lipid Res. 1998;37:33–72. doi: 10.1016/s0163-7827(98)00004-6. [DOI] [PubMed] [Google Scholar]
- Brown MR, Dunstan GA, Norwood SJ, Miller KA. Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J Phycol. 1996;32:64–73. [Google Scholar]
- Budge SM, Parrish CC. Lipid class and fatty acid composition of Pseudo-nitzschia multiseries and Pseudo-nitzschia pungens and effects of lipolytic enzyme deactivation. Phytochemistry. 1999;52:561–566. [Google Scholar]
- Chandra S, Heinstein PF, Low PS. Activation of phospholipase a by plant defense elicitors. Plant Physiol. 1996;110:979–986. doi: 10.1104/pp.110.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman KD. Phospholipase activity during plantgrowth and development and in response to environmental stress. Trends Plant Sci. 1998;3:419–426. [Google Scholar]
- Dunstan GA, Volkman JK, Barrett SM, Leroi JM, Jeffrey SW. Essential polyunsaturated fatty-acids from 14 species of diatom (bacillariophyceae) Phytochemistry. 1994;35:155–161. [Google Scholar]
- Feussner I, Kuhn H, Wasternack C. Lipoxygenase-dependent degradation of storage lipids. Trends Plant Sci. 2001;6:268–273. doi: 10.1016/s1360-1385(01)01950-1. [DOI] [PubMed] [Google Scholar]
- Gardner HW. Recent investigations into the lipoxygenase pathway of plants. Biochim Biophys Acta. 1991;1084:221–239. doi: 10.1016/0005-2760(91)90063-n. [DOI] [PubMed] [Google Scholar]
- Hause B, Weichert H, Hohne M, Kindl H, Feussner I. Expression of cucumber lipid-body lipoxygenase in transgenic tobacco: Lipid-body lipoxygenase is correctly targeted to seed lipid bodies. Planta. 2000;210:708–714. doi: 10.1007/s004250050671. [DOI] [PubMed] [Google Scholar]
- Ianora A, Poulet SA, Miralto A, Grottoli R. The diatom Thalassiosira rotula affects reproductive success in the copepod Acartia clausi. Mar Biol. 1996;125:279–286. [Google Scholar]
- Jüttner F. Liberation of 5,8,11,14,17-eicosapentaenoic acid and other polyunsaturated fatty acids from lipids as a grazer defense reaction in epilithic diatom biofilms. J Phycol. 2001;37:744–755. [Google Scholar]
- Jüttner F, Durst U. High lipoxygenase activities in epilithic biofilms of diatoms. Arch Hydrobiol. 1997;138:451–463. [Google Scholar]
- Kajiwara T, Hatanaka A, Matsui K, Tomoi T, Idohara T. Properties of a long-chain aldehyde-forming enzyme in the marine green-alga Ulva pertusa. Phytochemistry. 1994;35:55–57. [Google Scholar]
- Maier I, Calenberg M. Effect of extracellular Ca2+ and Ca2+-antagonists on the movement and chemoorientation of male gametes of Ectocarpus siliculosus (phaeophyceae) Bot Acta. 1994;107:451–460. [Google Scholar]
- Matsui K, Kurishita S, Hisamitsu A, Kajiwara T. A lipid-hydrolyzing activity involved in hexenal formation. Biochem Soc Trans. 2000;28:857–860. [PubMed] [Google Scholar]
- Minier C, Galgani F, Robert JM. In-vivo characterization of esterase-activity in calothrix pcc-7601, Haslea ostrearia and Prorocentrum micans. Bot Mar. 1993;36:245–252. [Google Scholar]
- Miralto A, Barone G, Romano G, Poulet SA, Ianora A, Russo GL, Buttino I, Mazzarella G, Laabir M, Cabrini M et al. The insidious effect of diatoms on copepod reproduction. Nature. 1999;402:173–176. [Google Scholar]
- Paul RU, Holk A, Scherer GFE. Fatty acids and lysophospholipids as potential second messengers in auxin action. Rapid activation of phospholipase a(2) activity by auxin in suspension-cultured parsley and soybean cells. Plant J. 1998;16:601–611. [Google Scholar]
- Pohnert G. Wound-activated chemical defense in unicellular planktonic algae. Angew Chem Int Edit. 2000;39:4352–4354. doi: 10.1002/1521-3773(20001201)39:23<4352::AID-ANIE4352>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Pohnert G, Boland W. Biosynthesis of the algal pheromone hormosirene by the freshwater diatom Gomphonema parvulum (bacillariophyceae) Tetrahedron. 1996;52:10073–10082. [Google Scholar]
- Roos W, Dordschbal B, Steighardt J, Hieke M, Weiss D, Saalbach G. A redox-dependent, G-protein-coupledphospholipase a of the plasma membrane is involved in the elicitation of alkaloid biosynthesis in Eschscholtzia californica. Biochim Biophys Acta Mol Cell Res. 1999;1448:390–402. doi: 10.1016/s0167-4889(98)00148-7. [DOI] [PubMed] [Google Scholar]
- Schiess K, Kaszkin M, Jordan P, Seidler L, Kinzel V. Mobilization of diacylglycerol in intact hela-cells by exogenous phospholipase-c from cl-perfringens is accompanied by release of fatty-acids including arachidonic-acid. Biochim Biophys Acta. 1992;1137:82–94. doi: 10.1016/0167-4889(92)90104-j. [DOI] [PubMed] [Google Scholar]
- Strosznajder J, Samochocki M. Ca2+-independent, Ca2+-dependent, and carbachol-mediated arachidonic-acid release from rat-brain cortex membrane. J Neurochem. 1991;57:1198–1206. doi: 10.1111/j.1471-4159.1991.tb08280.x. [DOI] [PubMed] [Google Scholar]
- Vollenweider S, Weber H, Stolz S, Chetelat A, Farmer EE. Fatty acid ketodienes and fatty acid ketotrienes: Michael addition acceptors that accumulate in wounded and diseased Arabidopsis leaves. Plant J. 2000;24:467–476. doi: 10.1046/j.1365-313x.2000.00897.x. [DOI] [PubMed] [Google Scholar]
- Wang XM. Plant phospholipases. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:211–231. doi: 10.1146/annurev.arplant.52.1.211. [DOI] [PubMed] [Google Scholar]
- Wolfe GV. The chemical defense ecology of marine unicellular plankton: constraints, mechanisms, and impacts. Biol Bull. 2000;198:225–244. doi: 10.2307/1542526. [DOI] [PubMed] [Google Scholar]
- Yongmanitchai W, Ward OP. Positional distribution of fatty-acids, and molecular-species of polar lipids, in the diatom Phaeodactylum tricornutum. J Gen Microbiol. 1993;139:465–472. doi: 10.1099/00221287-139-3-465. [DOI] [PubMed] [Google Scholar]