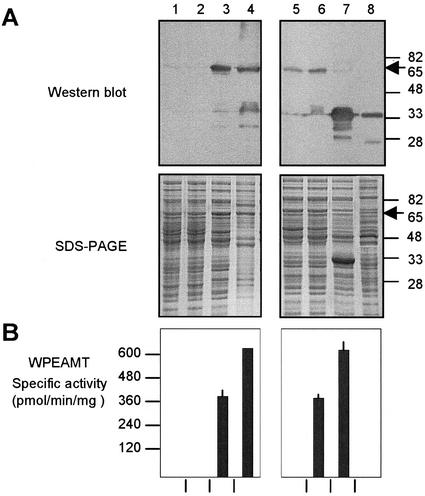
Figure 2.
Functional analysis of the recombinant WPEAMT. The WT WPEAMT, its mutants (M1 and M2), and deletions (H1 and H2) were expressed as N-terminal His tagged fusions in E. coli. All proteins (WT, M1, M2, and H1) were induced with isopropyl-β-d-thiogalactoside (IPTG) at 37°C for 3h except for H2, which was induced at 20°C for 16h. A, Upper, Immunoblot with the anti-WPEAMT antibodies. Lower, Protein pattern stained with Coomassie brilliant blue R-250. B, WPEAMT-specific activity using P-EA as substrate. The arrow indicates the location of the N-terminal fusion of the WT WPEAMT and mutants (65 kD). Lane 1, Untransformed E. coli; lane 2, transformed with WT WPEAMT before induction with IPTG; lane 3, WT WPEAMT after induction with IPTG; lane 4, purified WT WPEAMT on a His-bind resin column; lane 5, mutant M1 after induction; lane 6, mutant M2 after induction; lane 7, deletion H1 after induction; lane 8, deletion H2 after induction.