Abstract
Cytoplasmic sulfate for sulfation reactions may be derived either from extracellular fluids or from catabolism of sulfur-containing amino acids and other thiols. In vitro studies have pointed out the potential relevance of sulfur-containing amino acids as sources for sulfation when extracellular sulfate concentration is low or when its transport is impaired such as in DTDST [DTD (diastrophic dysplasia) sulfate transporter] chondrodysplasias. In the present study, we have considered the contribution of cysteine and cysteine derivatives to in vivo macromolecular sulfation of cartilage by using the mouse model of DTD we have recently generated [Forlino, Piazza, Tiveron, Della Torre, Tatangelo, Bonafe, Gualeni, Romano, Pecora, Superti-Furga et al. (2005) Hum. Mol. Genet. 14, 859–871]. By intraperitoneal injection of [35S]cysteine in wild-type and mutant mice and determination of the specific activity of the chondroitin 4-sulfated disaccharide in cartilage, we demonstrated that the pathway by which sulfate is recruited from the intracellular oxidation of thiols is active in vivo. To check whether cysteine derivatives play a role, sulfation of cartilage proteoglycans was measured after treatment for 1 week of newborn mutant and wild-type mice with hypodermic NAC (N-acetyl-L-cysteine). The relative amount of sulfated disaccharides increased in mutant mice treated with NAC compared with the placebo group, indicating an increase in proteoglycan sulfation due to NAC catabolism, although pharmacokinetic studies demonstrated that the drug was rapidly removed from the bloodstream. In conclusion, cysteine contribution to cartilage proteoglycan sulfation in vivo is minimal under physiological conditions even if extracellular sulfate availability is low; however, the contribution of thiols to sulfation becomes significant by increasing their plasma concentration.
Keywords: amino acid sulfur, cartilage, diastrophic dysplasia sulfate transporter, glycosaminoglycan, N-acetyl-L-cysteine, proteoglycan
Abbreviations: APS, adenosine-phosphosulfate; CHO cell, Chinese-hamster ovary cell; DMEM, Dulbecco's modified Eagle's medium; DTD, diastrophic dysplasia; DTDST, DTD sulfate transporter; DTPA, diethylenetriaminepenta-acetic acid; ΔDi-0S, 3-O-β-(D-gluc-4-ene-uronosyl)-N-acetylgalactosamine; ΔDi-4S, ΔDi-6S, derivatives of ΔDi-0S with a sulfate at position 4 or 6 of the hexosamine moiety, respectively; FCS, fetal calf serum; GAG, glycosaminoglycan; NAC, N-acetyl-L-cysteine; PAPS, adenosine 3′-phosphate 5′-phosphosulfate; RP-HPLC, reverse-phase HPLC; SEMD, spondyloepimetaphyseal dysplasia
INTRODUCTION
Sulfation occurs in all tissues and involves proteins, lipids, hormones and drugs. The control and degree of sulfation of such a wide range of substances suggest that this pathway is involved in many aspects of the life of an organism. Macromolecular sulfation is a post-translational event that is less studied compared with other post-translational modifications such as glycosylation or phosphorylation. Proteoglycans, the most known family of sulfated macromolecules, are present in the extracellular matrices and in the cell membranes; they are not only involved in structural and mechanical function, but also play more subtle roles, since they regulate diffusion of water and other compounds and are involved in cell differentiation and recognition [1].
Several sulfotransferases are involved in sulfation and their activity may be an important level at which regulation of sulfation occurs [2]; they show a strict acceptor specificity, but have the sulfate donor, PAPS (adenosine 3′-phosphate 5′-phosphosulfate), in common. Thus the availability of PAPS appears to be a limiting step for the sulfation activity of the cell. The intracellular availability of PAPS may itself be under multiple levels of control: (i) the activity of the bifunctional enzyme phosphoadenosine-phosphosulfate synthase [formerly ATP sulfurylase-APS (adenosine-phosphosulfate) kinase] which activates cytoplasmic sulfate to PAPS [3]; (ii) PAPS translocation across the membranes of the endoplasmic reticulum or the Golgi apparatus where the sulfotransferase reactions occur [4]; and (iii) the intracellular level of sulfate.
Genetic defects in the metabolism of sulfate causing generalized proteoglycan undersulfation have been described and produce clinical phenotypes of skeletal dysplasia [4a]. Mutations in the DTDST [DTD (diastrophic dysplasia) sulfate transporter; also known as SLC26A2 (solute carrier family 26 member 2)], a sulfate/chloride antiporter of the cell membrane, cause a spectrum of disorders ranging from lethal, generalized skeletal hypoplasia (achondrogenesis 1B and atelosteogenesis 2) to a non-lethal, short-stature dysplasia (DTD), to a relatively mild dysplasia with normal stature (recessive multiple epiphyseal dysplasia) ([5], but see [5a]). Mutations in an isoform of PAPS synthase (PAPSS2) cause a recessive form of SEMD (spondyloepimetaphyseal dysplasia) in humans (SEMD Pakistani type) [6] and brachymorphism in mice ([7], but see [7a]).
Inorganic sulfate in the cytoplasm may be derived either from the extracellular fluids or formed in the cytoplasm by catabolism of sulfur-containing amino acids (cysteine and methionine) and other thiols. Cysteine is oxidized to cysteine-sulfinate, which can then undergo transamination to sulfinylpyruvate which spontaneously decomposes to pyruvate and sulfite. Sulfite is then oxidized to sulfate by sulfite oxidase, an enzyme present in many tissues. Methionine degradation occurs through its conversion at first into cysteine.
The contribution of sulfur-containing compounds other than inorganic sulfate to macromolecular sulfation varies in different cell systems. Chondrocytes and endothelial cells appear to be mostly dependent on extracellular sulfate [8,9], whereas CHO cells (Chinese-hamster ovary cells) make more extensive use of cysteine-derived sulfate [10]. In fibroblasts, both pathways are active [11,12]; in addition, it has been demonstrated in lung fibroblasts that cysteine can be a major source of sulfate at low extracellular sulfate concentration (below 100 μM) [11].
All these studies have raised awareness to the potential importance of sulfur-containing amino acids as sources for sulfation when extracellular sulfate concentration is low or when its transport is impaired such as in DTDST chondrodysplasias [13]. We have previously demonstrated that sulfate recruitment from sulfur-containing amino acids is active in cultured fibroblasts and chondrocytes from DTDST patients and this pathway, at least in vitro, can partially compensate for the lack of sulfate caused by the reduced uptake function of the sulfate transporter [14,15]. However, no data are available regarding the function of this pathway in cartilage in vivo.
In order to study in vivo the contribution of sulfur-containing compounds to the intracellular sulfate pool, extracellular sulfate availability has been reduced by decreasing dietary sulfate or using molybdate, which inhibits sulfate intestinal absorption and renal re-adsorption, but impairs also sulfate incorporation into APS during the first step of PAPS synthesis [3].
We have recently generated a mouse model of DTD (dtd mouse) by ‘knocking’ into the murine Dtdst gene the A386V substitution; our results demonstrated that the mouse model reproduces human DTD at the morphological and biochemical levels [16]. This animal model is a valuable tool to assess in vivo the contribution of sulfur-containing amino acids to macromolecular sulfation when intracellular sulfate availability is low. Thus using the dtd mouse we have determined in vivo the contribution of cysteine and cysteine derivatives to macromolecular sulfation of cartilage, a tissue with high sulfate requirement.
MATERIALS AND METHODS
Chemicals
6-3H]Glucosamine and [35S]cysteine were purchased from Amersham Biosciences Europe (Milan, Italy). Papain, 1-octanesulfonic acid, sodium nitrite and N,N-dimethylformamide were from Sigma–Aldrich (Milan, Italy). NAC (N-acetyl-L-cysteine), 2-mercaptoethanol, 5-sulfosalicylic acid, DTPA (diethylenetriaminepenta-acetic acid), tributylphosphine and acetonitrile (HPLC grade) were from Fluka (Sigma–Aldrich, Milan, Italy). Streptomyces hyaluronidase and chondroitinase ABC and ACII were obtained from Seikagaku (Tokyo, Japan).
Mouse strain
The mouse model used in the present study is a ‘knock-in’ mouse homozygous for a c1184t transition causing an A386V substitution in the DTDST gene [16]. This mutation was detected in the homozygous state in a patient with a moderate form of DTD characterized by short stature, cleft palate, deformity of external ear and ‘hitchhiker’ thumb deformity [16]. Animals were bred with free access to water and standard pelleted food. Experimental animal procedures were approved by local and national authorities.
Metabolic labelling of cartilage explants with [35S]cysteine and [3H]glucosamine
Cartilage used for these studies comes from the femoral head, which, in mice, is completely cartilaginous in the first week of age. The femoral heads from newborn dtd and wild-type mice were recovered and cultured for 24 h in DMEM (Dulbecco's modified Eagle's medium) containing 10% FCS (fetal calf serum) at 37 °C in 5% CO2. Cartilage explants were metabolically labelled with [6-3H]glucosamine and [35S]cysteine in DMEM containing 25 μM cystine and methionine, 250 μM Na2SO4 and 5% dialysed FCS at 37 °C for 24 h. At the end of the labelling period, the medium was removed and cartilage explants were digested with papain in 0.1 M sodium acetate (pH 5.6), 5 mM cysteine and 5 mM EDTA at 65 °C for 24 h. GAGs (glycosaminoglycans) were then recovered by cetylpyridinium chloride precipitation. Hyaluronic acid was removed by digestion with Streptomyces hyaluronidase and digestion products were removed by ultrafiltration (Biomax Ultrafree-0.5; Millipore). GAGs in the non-diffusible material were digested at 37 °C overnight with 30 m-units each of chondroitinase ABC and ACII in 30 mM Tris-acetate and 30 mM sodium acetate (pH 7.35). Undigested products were removed by precipitation with 4 vol. of ethanol; chondroitin sulfate disaccharides in the supernatant were then analysed by HPLC with a Supelcosil LC-SAX1 column (Supelco) as previously described [16]. Standard unsaturated disaccharides {ΔDi-0S [3-O-β-(D-gluc-4-ene-uronosyl)-N-acetylgalactosamine] and ΔDi-4S and ΔDi-6S (derivatives of ΔDi-0S with a sulfate at position 4 or 6 of the hexosamine moiety)} were added to radiolabelled samples and the corresponding peaks at 232 nm were collected and counted for 35S and 3H radioactivity.
In vivo metabolic labelling with [35S]cysteine
Two-day-old pups were injected intraperitoneally with 25 μCi/g body weight of [35S]cysteine at high specific activity (>1000 Ci/mmol). After a labelling period of 24 h, pups were killed and chondroitin sulfate proteoglycan sulfation from cartilage of the femoral heads and from skin was measured. Briefly, tissue biopsies were digested with papain, hyaluronic acid was removed by digestion with hyaluronidase and GAGs were then digested with chondroitinase ABC and ACII. Chondroitin sulfate disaccharides were then separated by HPLC with a Supelcosil LC-SAX1 column (Supelco) as described above. The peak corresponding to the chondroitin 4-sulfated disaccharide (ΔDi-4S) was collected and radioactivity was determined by liquid-scintillation counting.
Hypodermic administration of NAC and pharmacokinetic studies
Pups at 1 day of age were daily treated with hypodermic injection of 1 g/kg body weight of NAC for 7 days. At the end of the treatment, the pups were killed and chondroitin sulfate proteoglycan sulfation from cartilage of the femoral heads was measured as described above.
For pharmacokinetic studies, wild-type pups at 4 days of age were injected with 1 g/kg body weight of NAC. Pups were killed at 0, 2, 4, 8 and 24 h after injection, and blood was collected in tubes containing EDTA and immediately centrifuged at 4 °C (800 g, 5 min). Plasma samples, after the addition of 2-mercaptoethanol as an internal standard, were treated with 10% (v/v) tributylphosphine in N,N-dimethylformamide [2% (v/v) final concentration] for 30 min at 4 °C in order to reduce thiols and release them from plasma proteins [17,18]. Subsequently, a 50% solution of 5-sulfosalicylic acid and 1 mM EDTA was added to each sample to a final concentration of 10% and 0.2 mM respectively. Samples were then centrifuged in order to discard proteins and a solution of 100 mM DTPA, 10 mM sodium nitrite and 5 M HCl was added to supernatants to final concentrations of 10 mM DTPA, 1 mM sodium nitrite and 0.5 M HCl. For quantitative measurements, samples were allowed to stand at room temperature (20 °C) for 20 min for maximum derivative formation. Each sample was finally centrifuged for 5 min at 18000 g and the supernatant was analysed by RP-HPLC (reverse-phase HPLC) with a C18 endcapped Superspher 100 column (250 mm×4.6 mm; Merck) [19]. The mobile phases were: buffer A: aqueous solution of 10 mM sodium dihydrogenphosphate and 10 mM 1-octanesulfonic acid as cation-pairing, brought to pH 2.0 with concentrated H3PO4; buffer B: solution of water/acetonitrile (50:50, v/v) containing 10 mM sodium dihydrogenphosphate and 10 mM 1-octanesulfonic acid, brought to pH 2.0 with concentrated H3PO4. Gradient elution from 0 to 40% of buffer B in 45 min was performed at a flow rate of 1 ml/min and the eluate was monitored at 333 nm. Standard curves were prepared by derivative formation and RP-HPLC analyses of mixtures of NAC (range 3–30 nmol), cysteine and 2-mercaptoethanol (range 1–10 nmol).
Statistical analysis
Statistical differences between wild-type and mutant groups were determined by Student's t test; P<0.05 was considered significant.
RESULTS
Metabolic labelling of cartilage organ cultures
To test the hypothesis that oxidation of sulfur-containing amino acids (mainly cysteine) can provide sulfate for proteoglycan sulfation in mice, as already observed in cultured chondrocytes from DTDST patients [14], the femoral heads from newborn wild-type and dtd mice were harvested, cultured for 2 days in DMEM containing 10% FCS and then labelled with [3H]glucosamine and [35S]cysteine for 24 h. GAGs were purified from labelled cartilage by papain digestion and β-elimination to remove completely the core protein and hyaluronidase digestion to remove hyaluronic acid; their 35S/3H ratio was then determined. The ratio was 4-fold higher in dtd mice compared with wild-type animals, demonstrating that sulfate recruitment from amino acid sulfur was active also in mice (0.415±0.004 versus 0.109±0.013; P<0.001, n=4). In the same experiment, sulfation of newly synthesized chondroitin/dermatan sulfate GAGs was also measured. For this purpose, purified GAGs were digested with chondroitinase ABC and ACII and released disaccharides were separated by HPLC and counted for 3H activity. In dtd mice, the relative amount of non-sulfated disaccharide was increased compared with wild-types, indicating that newly synthesized proteoglycans were undersulfated (Table 1); interestingly, the extent of proteoglycan undersulfation in vitro was similar to the values observed ex vivo in newborn dtd animals [16]. The increased 35S/3H ratio observed in GAGs from dtd mice was confirmed when the same ratio was measured in mono-sulfated disaccharides from chondroitin/dermatan sulfate chains (Table 1).
Table 1. Chondroitin sulfate disaccharide analysis in newly synthesized proteoglycans from cartilage organ cultures.
Cartilage slices from wild-type and dtd mice were metabolically labelled with [35S]cysteine and [3H]glucosamine; GAGs were then purified and digested with chondroitinase ABC and ACII and released disaccharides were separated by HPLC and counted for 3H activity. The relative amount of non-sulfated disaccharide (ΔDi-0S) in dtd mice was significantly increased compared with wild-type mice, indicating proteoglycan undersulfation. At the same time, the 35S/3H ratio in mono-sulfated disaccharides was measured and values demonstrated that sulfur from cysteine catabolism can provide sulfate for proteoglycan sulfation as observed in GAGs. Results shown are the means±S.D. for cartilage organ cultures from four dtd and wild-type mice. *P<0.05; **P<0.001.
| Disaccharide (%)* | 35S/3H** | ||||
|---|---|---|---|---|---|
| ΔDi-0S | ΔDi-4S | ΔDi-6S | ΔDi-4S | ΔDi-6S | |
| dtd | 42.25±4.58 | 55.75±4.30 | 1.99±0.34 | 0.614±0.046 | 0.841±0.056 |
| Wild-type | 11.22±0.48 | 86.10±0.68 | 2.68±0.27 | 0.110±0.015 | 0.124±0.025 |
In vivo labelling with [35S]cysteine
The experiments described above, as well as those reported previously [14,15], targeted at demonstrating sulfate recruitment from cysteine catabolism, have been performed in cultured chondrocytes. However, chondrocytes easily dedifferentiate in culture; therefore it has been necessary to confirm whether the catabolism of sulfur-containing amino acid is active also in vivo. For this purpose, [35S]cysteine was injected intraperitoneally into 2-day-old dtd and wild-type mice. The study was performed with labelled cysteine at a high specific activity (>1000 Ci/mmol) in order to preserve the physiological concentration of plasma cysteine which is in the micromolar range. To measure the contribution of sulfur from cysteine catabolism to proteoglycan sulfation, the specific activity of ΔDi-4S was measured in cartilage and in skin. ΔDi-4S was selected because it is the mono-sulfated disaccharide most represented among chondroitin sulfate disaccharides. For this purpose, after 24 h labelling, cartilage from the femoral heads and skin were digested with papain to remove the core proteins and purified GAGs were digested with chondroitinase ABC and ACII. Disaccharides were analysed by HPLC and the amount of 35S radioactivity associated with ΔDi-4S was measured. The specific activity (dpm/nmol) was normalized to the body weight of the animals and to blood radioactivity as an index of absorbed [35S]cysteine available to tissues. In both tissues, 35S activity was associated with ΔDi-4S, indicating that the pathway of sulfate recruitment from the catabolism of sulfur-containing amino acids was active in vivo. In particular, the specific activity was higher in cartilage and skin from dtd animals compared with wild-types (Table 2).
Table 2. Specific activity of ΔDi-4S from cartilage after in vivo labelling with [35S]cysteine.
Labelled cysteine was injected in dtd and wild-type mice; after 24 h, mice were killed and GAGs from cartilage of the femoral heads and from skin were purified for disaccharide analysis. After digestion with chondroitinase ABC and ACII, disaccharides were analysed by HPLC and the amount of 35S activity associated with ΔDi-4S was measured. The specific activity (dpm/nmol) was normalized to blood radioactivity and to the body weight of the animals. The results shown, which are the means±S.D. for four separate mice, demonstrate that sulfate recruitment from the catabolism of cysteine is active in cartilage in vivo. *P<0.05.
| Specific activity (dpm/nmol)/(dpm in blood/body weight) | ||
|---|---|---|
| Cartilage* | Skin | |
| dtd | 0.0161±0.0018 | 0.0449±0.0197 |
| Wild-type | 0.0101±0.0012 | 0.0218±0.0068 |
NAC contribution to proteoglycan sulfation
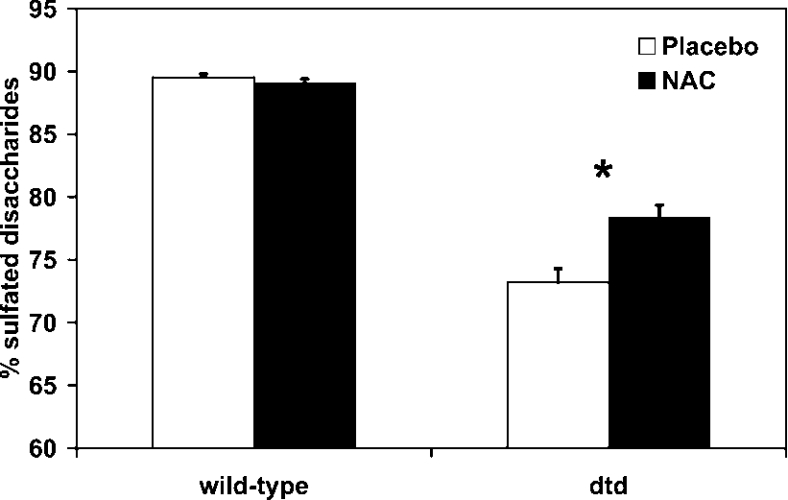
On the basis of in vivo data reported above, we checked the hypothesis whether cysteine derivatives can contribute to the intracellular sulfate pool and thus play a role in proteoglycan sulfation when extracellular sulfate availability is reduced as in dtd mice. For this purpose, newborn dtd and wild-type mice received hypodermic injection of NAC (1 g/kg body weight) in 5% (w/v) glucose every 24 h for 7 days; the placebo group was treated with 5% glucose. At the end of the treatment, the animals were killed and proteoglycan sulfation of cartilage from the femoral heads was measured. In wild-type animals, no differences were observed in proteoglycan sulfation in the group treated with NAC compared with the placebo, the relative amount of mono-sulfated disaccharides (ΔDi-4S and ΔDi-6S) was the same (89%). In dtd animals treated with the placebo, the relative amount of monosulfated disaccharides was lower compared with the wild-types (73% versus 89%), indicating undersulfation of proteoglycans as a consequence of decreased extracellular sulfate uptake caused by the mutation in the Dtdst. In dtd animals treated with hypodermic NAC, the relative amount of mono-sulfated disaccharides increased compared with the placebo group (78% versus 73%, P<0.05), indicating an increase in proteoglycan sulfation due to NAC catabolism (Figure 1).
Figure 1. Contribution of NAC to cartilage proteoglycan sulfation.
Newborn dtd and wild-type mice received daily hypodermic NAC for 7 days and then cartilage proteoglycan sulfation was measured by HPLC disaccharide analysis on the basis of the relative amount of sulfated disaccharides versus total disaccharides. As expected, in wild-type mice treated either with NAC or placebo, proteoglycan sulfation was not affected, whereas in dtd mice, NAC contributed, through its catabolism, to proteoglycan sulfation. The increase was weak, but the difference between the values in the dtd group treated with the placebo compared with the one treated with NAC was statistically significant (*P<0.05; n=7).
Pharmacokinetics of hypodermic NAC
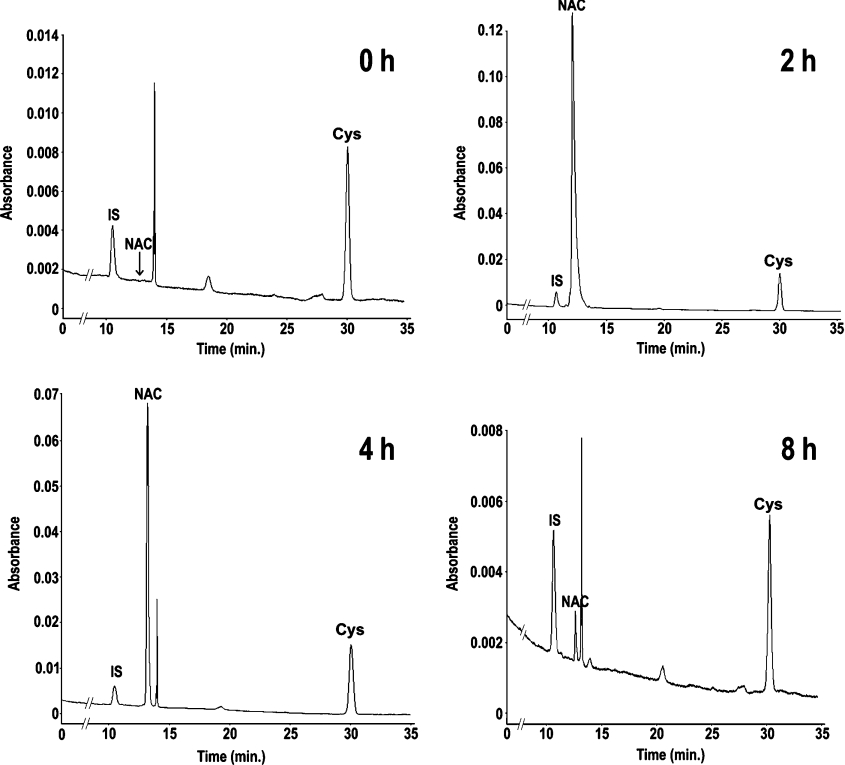
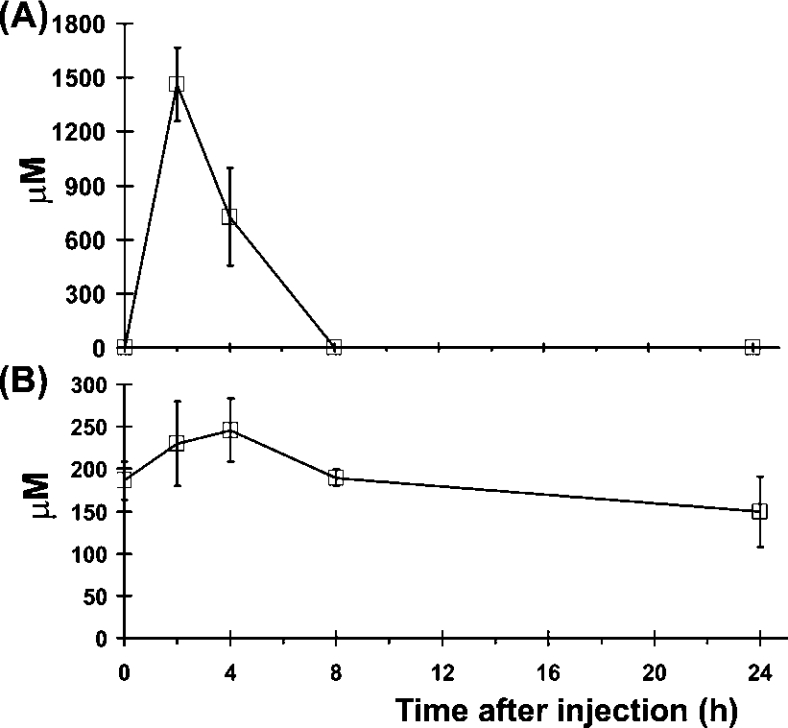
The increase in cartilage proteoglycan sulfation in dtd animals treated with NAC was significant but weak with respect to the placebo group. To check whether the reduced efficacy of NAC treatment in cartilage sulfation was due to reduced cartilage vascularization, which impairs the availability of the drug to chondrocytes, or instead due to a rapid NAC catabolism, we performed a pharmacokinetic study of hypodermic NAC. For this purpose, 4-day-old wild-type mice were injected with NAC (1 g/kg body weight) in 5% glucose; at different time intervals (0, 2, 4, 8 and 24 h), animals were killed and the concentrations of plasma NAC and cysteine were measured. To measure the plasma level of NAC and cysteine in micro-samples (50–70 μl) of plasma, we set up an HPLC method with some modifications from previously published methods [19]. Briefly, to measure total NAC and cysteine (not oxidized and oxidized), plasma samples were reduced with tributylphosphine, proteins were removed by sulfosalicylic acid precipitation and thiols in the supernatant were derivatized with nitrous acid. The derivatized products were stabilized with DTPA and then analysed by RP-HPLC. Plasma chromatograms at different time points after administration of NAC are shown in Figure 2. Under normal conditions, NAC is not present in plasma; after subcutaneous administration of 1 g/kg NAC, the maximum plasma concentration was observed after 2 h (1460 μM), then NAC level rapidly decreased to trace amount within 8 h from injection; after 24 h, no NAC was detected in plasma (Figure 3A). To check whether NAC also affects cysteine availability, we measured the plasma concentration of cysteine after NAC injection. A slight increase in cysteine concentration corresponding to the increase in NAC concentration was observed; however, it was not statistically significant (Figure 3B). In conclusion, hypodermic NAC was removed from the bloodstream within 8 h and under these conditions it does not affect cysteine plasma concentration.
Figure 2. Representative RP-HPLC analysis of plasma from wild-type mice.
Wild-type pups received hypodermic cysteine and the plasma concentration of NAC and cysteine was measured at 0 h (upper left panel), 2 h (upper right panel), 4 h (lower left panel) and 8 h (lower right panel) from injection. For this purpose, the plasma was derivatized with nitrous acid and S-nitroso derivatives of 2-mercaptoethanol as internal standards (IS); NAC and cysteine (Cys) were separated by RP-HPLC. Note that at 0 and 8 h from injection, NAC is present in trace amount.
Figure 3. Plasma pharmacokinetics of hypodermic NAC.
Plasma levels of NAC and cysteine after hypodermic injection of 1 g of NAC/kg body weight were monitored over 24 h using the HPLC method shown in Figure 2. The maximal concentration of NAC is observed at 2 h from injection (A); interestingly, after 8 h, NAC is present only in trace amount, indicating that NAC is rapidly removed from the bloodstream. A slight increase in the plasma level of cysteine was observed after 4 h; however, differences were not statistically significant (B). Results are expressed as means±S.D. (n=4).
DISCUSSION
Sulfation is the transfer of a sulfate group to different substrates. PAPS is the obligate donor for the sulfotransferase reaction and its synthesis is dependent on the availability of intracellular sulfate, which, in turn, relies mainly on sulfate transport from the extracellular space and sulfoxidation of sulfur-containing amino acids. Under normal condition, sulfate released from the lysosomal degradation of GAGs rapidly exchanges with sulfate in the extracellular space and does not appear to be appreciably recycled [20,21]. The contribution of extracellular sulfate and sulfur amino acids to the intracellular sulfate pool varies in different cell systems: chondrocytes and endothelial cells in vitro are mostly dependent on extracellular sulfate for macromolecular sulfation, whereas CHO cells make extensive use of cysteine-derived sulfate [8–10]. Indirect evidence of the minor contribution of amino acid sulfur to the sulfate pool of cartilage comes from studies of DTDST chondrodysplasias, a group of disorders that are caused by sulfate uptake impairment; under this condition, undersulfation of cartilage proteoglycans is observed, demonstrating that in this tissue at normal concentrations of sulfate and cysteine the intracellular sulfate pool relies mainly on extracellular sulfate.
The experimental approaches to study the role of amino acid sulfur in the intracellular sulfate pool in vivo are more complicated. Sulfate availability has been reduced by decreasing dietary sulfate or using molybdate which inhibits sulfate intestinal and renal adsorption, but also affects the PAPS reaction [3]. We have recently generated a mouse model of DTD, a chondrodysplasia caused by mutations in the DTDST gene that cause sulfate uptake impairment. This mouse model offers a valuable approach to study in vivo the contribution of sulfur-containing amino acids to the sulfate pool when sulfate availability is reduced. By using the dtd mouse, we have focused on the activity of this alternative pathway of sulfate recruitment in cartilage, the tissue mostly affected in DTDST chondrodysplasias. We have already demonstrated that this pathway is active in vitro using chondrocytes from DTD patients [14]; however, due to the different environment of chondrocytes in culture with respect to the tissue and to the ease of dedifferentiation of chondrocytes, the situation in vivo could be different.
Among sulfur-containing amino acids, we considered cysteine since several authors have reported in different cell types that methionine's contribution to the PAPS pool is 6–10 times lower than that of cysteine [10,11,22]. In addition, it has been demonstrated also that infusion of methionine is less effective than cysteine in increasing tissue sulfate concentration in rats [23]. These findings seem reasonable considering that methionine is first metabolized to cysteine before its oxidation.
It has been reported that there are not only differences in the sulfation capacity among tissues, but also differences among species in the factors that account for limited sulfation capacity [3]. For this reason, we have first checked whether cysteine catabolism is active also in murine chondrocytes as already observed in humans [14]. By double labelling of cartilage slices of wild-type and dtd animals with [35S]cysteine and [3H]glucosamine, we demonstrated that this pathway is active also in mice. To demonstrate that sulfur-containing amino acids contribute to the intracellular sulfate pool in vivo, [35S]cysteine was injected in newborn dtd and wild-type mice and the specific activity of ΔDi-4S was measured. 35S activity associated with the mono-sulfated disaccharide demonstrated that this pathway was also active in vivo. At physiological concentrations of cysteine, the low specific activity in wild-type animals demonstrated that sulfation from amino acid catabolism is a minor pathway contributing to the intracellular sulfate pool of cartilage. The increased specific activity in dtd animals was interpreted to be due to an increase in the intracellular specific activity of sulfate as a result of sulfate production by cysteine oxidation and its mixing in a common intracellular pool. Since, in fibroblasts, it is reported that cysteine contributes significantly to the intracellular pool of sulfate when the extracellular concentration of sulfate is low (<100 μM) [11], we increased the plasma concentration of thiols in order to check whether their catabolism might be increased consequently. The concentration of cysteine is tightly regulated by the liver to keep intracellular cysteine concentration below the threshold of cytotoxicity [24]. The toxicity of cysteine has been demonstrated in animal models [25], whereas, in humans, it has been associated with Parkinson's and Alzheimer's diseases [26] or with increased risk of cardiovascular disease [27]; in addition, cysteine is reported to be poorly soluble. Thus we used NAC as a source of thiols since we have reported previously that this compound can increase proteoglycan sulfation in fibroblasts and chondrocytes from DTDST patients [15]; in addition, NAC has a positive record of low toxicity and has been studied extensively in the clinical environment [28,29]. The contribution of thiol catabolism to the intracellular pool of sulfate was evaluated on the basis of proteoglycan sulfation in dtd mice. The studies were performed in newborns since at this age we observed that cartilage proteoglycan undersulfation is maximal [16]; thus differences in proteoglycan sulfation due to sulfur amino acid catabolism are more evident. Owing to the young age of the mice, the only way of administration was daily hypodermic injection of NAC for 1 week; after the treatment, animals were killed and the level of proteoglycan sulfation was measured by HPLC disaccharide analysis. In the group of dtd animals treated with NAC, there was a weak, but statistically significant, increase in proteoglycan sulfation compared with the placebo. These results demonstrate that amino acid catabolism contributes to the intracellular sulfate pool when extracellular sulfate availability is low; furthermore, NAC does not affect proteoglycan sulfation in wild-type animals. The findings in pharmacokinetic experiments that NAC is rapidly removed from the bloodstream within 8 h suggest that its contribution might be even higher if the daily plasma level was more constant.
In conclusion, we have demonstrated in vivo that cysteine contribution to cartilage proteoglycan sulfation is minimal at physiologic concentrations of cysteine even if extracellular sulfate availability is low; however, the contribution of thiol compounds to proteoglycan sulfation becomes significant by increasing their plasma concentration.
Acknowledgments
We thank Angelo Gallanti (Department of Biochemistry, University of Pavia, Pavia, Italy) for expert cell culturing. This work was supported by grants from the Comitato Telethon Fondazione ONLUS (Rome, Italy) and Fondazione Cariplo (Milan, Italy).
References
- 1.Iozzo R. V. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 2.Habuchi O. Diversity and functions of glycosaminoglycan sulfotransferases. Biochim. Biophys. Acta. 2000;1474:115–127. doi: 10.1016/s0304-4165(00)00016-7. [DOI] [PubMed] [Google Scholar]
- 3.Klaassen C. D., Boles J. W. Sulfation and sulfotransferases 5: the importance of 3′-phosphoadenosine 5′-hosphosulfate (PAPS) in the regulation of sulfation. FASEB J. 1997;11:404–418. doi: 10.1096/fasebj.11.6.9194521. [DOI] [PubMed] [Google Scholar]
- 4.Hirschberg C. B., Robbins P. W., Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu. Rev. Biochem. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]
- 4a.Superti-Furga A. Defects in sulfate metabolism and skeletal dysplasias. In: Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Vogelstein B., Childs B., editors. The Metabolic and Molecular Bases of Inherited Disease. 8th edn. New York: McGraw-Hill; 2001. pp. 5189–5201. [Google Scholar]
- 5.Rossi A., Superti-Furga A. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene (SLC26A2): 22 novel mutations, mutation review, associated skeletal phenotypes, and diagnostic relevance. Hum. Mutat. 2001;17:159–171. doi: 10.1002/humu.1. [DOI] [PubMed] [Google Scholar]
- 5a.Erratum. Hum. Mutat. 2001;18:82. [Google Scholar]
- 6.ul Haque M. F., King L. M., Krakow D., Cantor R. M., Rusiniak M. E., Swank R. T., Superti-Furga A., Haque S., Abbas H., Ahmad W., et al. Mutations in orthologous genes in human spondyloepimetaphyseal dysplasia and the brachymorphic mouse. Nat. Genet. 1998;20:157–162. doi: 10.1038/2458. [DOI] [PubMed] [Google Scholar]
- 7.Kurima K., Warman M. L., Krishnan S., Domowicz M., Krueger R. C., Jr, Deyrup A., Schwartz N. B. A member of a family of sulfate-activating enzymes causes murine brachymorphism. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8681–8685. doi: 10.1073/pnas.95.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Erratum. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12071. [Google Scholar]
- 8.Ito K., Kimata K., Sobue M., Suzuki S. Altered proteoglycan synthesis by epiphyseal cartilages in culture at low SO42− concentration. J. Biol. Chem. 1982;257:917–923. [PubMed] [Google Scholar]
- 9.Humphries D. E., Silbert C. K., Silbert J. E. Sulfation by cultured cells. Cysteine, cysteinesulfinic acid and sulfite as sources for proteoglycan sulfate. Biochem. J. 1988;252:305–308. doi: 10.1042/bj2520305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esko J. D., Elgavish A., Prasthofer T., Taylor W. H., Weinke J. L. Sulfate transport-deficient mutants of Chinese hamster ovary cells. Sulfation of glycosaminoglycans dependent on cysteine. J. Biol. Chem. 1986;261:15725–15733. [PubMed] [Google Scholar]
- 11.Elgavish A., Meezan E. Sulfation by human lung fibroblasts: SO42− and sulfur-containing amino acids as sources for macromolecular sulfation. Am. J. Physiol. 1991;260:L450–L456. doi: 10.1152/ajplung.1991.260.6.L450. [DOI] [PubMed] [Google Scholar]
- 12.Keller J. M., Keller K. M. Amino acid sulfur as a source of sulfate for sulfated proteoglycans produced by Swiss mouse 3T3 cells. Biochim. Biophys. Acta. 1987;926:139–144. doi: 10.1016/0304-4165(87)90230-3. [DOI] [PubMed] [Google Scholar]
- 13.Hästbacka J., de la C. A., Mahtani M. M., Clines G., Reeve Daly M. P., Daly M., Hamilton B. A., Kusumi K., Trivedi B., Weaver A., et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 14.Rossi A., Bonaventure J., Delezoide A. L., Superti-Furga A., Cetta G. Undersulfation of cartilage proteoglycans ex vivo and increased contribution of amino acid sulfur to sulfation in vitro in McAlister dysplasia/atelosteogenesis type 2. Eur. J. Biochem. 1997;248:741–747. doi: 10.1111/j.1432-1033.1997.t01-1-00741.x. [DOI] [PubMed] [Google Scholar]
- 15.Rossi A., Cetta A., Piazza R., Bonaventure J., Steinmann B., Superti-Furga A. In vitro proteoglycan sulfation derived from sulfhydryl compounds in sulfate transporter chondrodysplasias. Pediatr. Pathol. Mol. Med. 2003;22:311–321. doi: 10.1080/pdp.22.4.311.321. [DOI] [PubMed] [Google Scholar]
- 16.Forlino A., Piazza R., Tiveron C., Della Torre S., Tatangelo L., Bonafe L., Gualeni B., Romano A., Pecora F., Superti-Furga A., et al. A diastrophic dysplasia sulfate transporter (SLC26A2) mutant mouse: morphological and biochemical characterization of the resulting chondrodysplasia phenotype. Hum. Mol. Genet. 2005;14:859–871. doi: 10.1093/hmg/ddi079. [DOI] [PubMed] [Google Scholar]
- 17.Rizzo V., Montalbetti L., Valli M., Bosoni T., Scoglio E., Moratti R. Study of factors affecting the determination of total plasma 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate (SBD)-thiol derivatives by liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1998;706:209–215. doi: 10.1016/s0378-4347(97)00547-1. [DOI] [PubMed] [Google Scholar]
- 18.Accinni R., Campolo J., Bartesaghi S., De Leo G., Lucarelli C., Cursano C. F., Parodi O. High-performance liquid chromatographic determination of total plasma homocysteine with or without internal standards. J. Chromatogr. A. 1998;828:397–400. doi: 10.1016/s0021-9673(98)00661-x. [DOI] [PubMed] [Google Scholar]
- 19.Tsikas D., Sandmann J., Ikic M., Fauler J., Stichtenoth D. O., Frolich J. C. Analysis of cysteine and N-acetylcysteine in human plasma by high-performance liquid chromatography at the basal state and after oral administration of N-acetylcysteine. J. Chromatogr. B Biomed. Sci. Appl. 1998;708:55–60. doi: 10.1016/s0378-4347(97)00670-1. [DOI] [PubMed] [Google Scholar]
- 20.Rome L. H., Hill D. F. Lysosomal degradation of glycoproteins and glycosaminoglycans. Efflux and recycling of sulfate and N-acetylhexosamines. Biochem. J. 1986;235:707–713. doi: 10.1042/bj2350707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper G. S., Rozaklis T., Bielicki J., Hopwood J. J. Lysosomal sulfate efflux following glycosaminoglycan degradation: measurements in enzyme-supplemented Maroteaux-Lamy syndrome fibroblasts and isolated lysosomes. Glycoconj. J. 1993;10:407–415. doi: 10.1007/BF00731045. [DOI] [PubMed] [Google Scholar]
- 22.Imai Y., Yanagishita M., Hascall V. C. Measurement of contribution from intracellular cysteine to sulfate in phosphoadenosine phosphosulfate in rat ovarian granulosa cells. Arch. Biochem. Biophys. 1994;312:392–400. doi: 10.1006/abbi.1994.1324. [DOI] [PubMed] [Google Scholar]
- 23.Kim H. J., Madhu C., Cho J. H., Klaassen C. D. In vivo modification of 3′-phosphoadenosine 5′-phosphosulfate and sulfate by infusion of sodium sulfate, cysteine, and methionine. Drug Metab. Dispos. 1995;23:840–845. [PubMed] [Google Scholar]
- 24.Dominy J. E., Jr, Hirschberger L. L., Coloso R. M., Stipanuk M. H. Regulation of cysteine dioxygenase degradation is mediated by intracellular cysteine levels and the ubiquitin-26 S proteasome system in the living rat. Biochem. J. 2006;394:267–273. doi: 10.1042/BJ20051510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann A., Hagberg H., Orwar O., Sandberg M. Cysteine sulfinate and cysteate: mediators of cysteine toxicity in the neonatal rat brain? Eur. J. Neurosci. 1993;5:1398–1412. doi: 10.1111/j.1460-9568.1993.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 26.Heafield M. T., Fearn S., Steventon G. B., Waring R. H., Williams A. C., Sturman S. G. Plasma cysteine and sulfate levels in patients with motor neurone, Parkinson's and Alzheimer's disease. Neurosci. Lett. 1990;110:216–220. doi: 10.1016/0304-3940(90)90814-p. [DOI] [PubMed] [Google Scholar]
- 27.Ozkan Y., Ozkan E., Simsek B. Plasma total homocysteine and cysteine levels as cardiovascular risk factors in coronary heart disease. Int. J. Cardiol. 2002;82:269–277. doi: 10.1016/s0167-5273(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 28.Iwata F., Kuehl E. M., Reed G. F., McCain L. M., Gahl W. A., Kaiser-Kupfer M. I. A randomized clinical trial of topical cysteamine disulfide (cystamine) versus free thiol (cysteamine) in the treatment of corneal cystine crystals in cystinosis. Mol. Genet. Metab. 1998;64:237–242. doi: 10.1006/mgme.1998.2725. [DOI] [PubMed] [Google Scholar]
- 29.Moldeus P., Cotgreave I. A. N-Acetylcysteine. Methods Enzymol. 1994;234:482–492. doi: 10.1016/0076-6879(94)34119-2. [DOI] [PubMed] [Google Scholar]