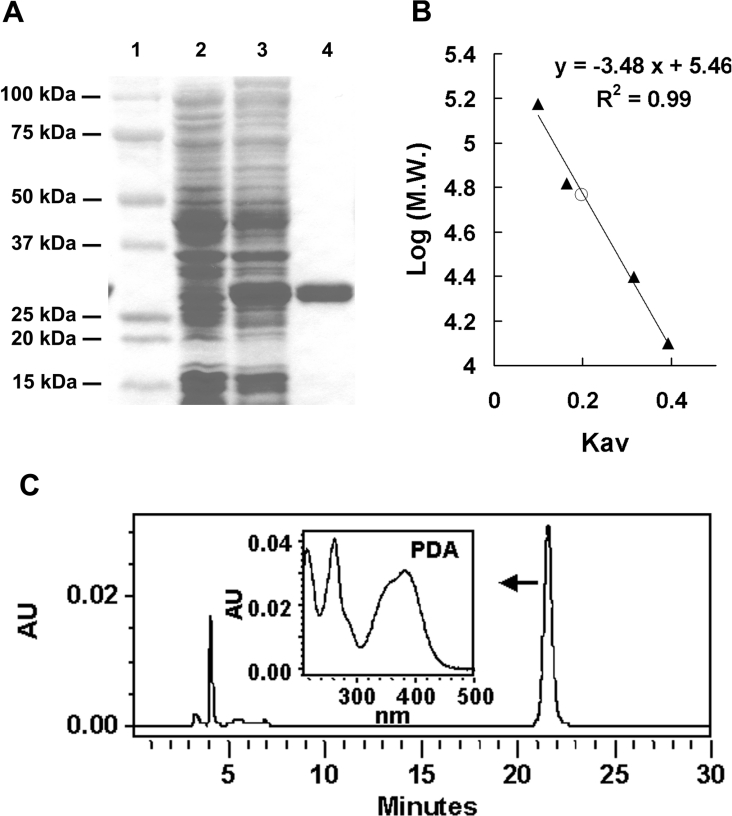
Figure 3. Molecular mass and PDA synthase activity of the CG6781 product.
(A) Recombinant CG6781, purified with His·Bind resin and subjected to electrophoresis on an SDS/4–20% acrylamide gradient gel, has a molecular mass of approx. 28 kDa. Molecular masses of standards are indicated. 1, standard markers; 2, crude extract before induction; 3, crude extract after induction; 4, purified enzyme. (B) The native molecular mass of the enzyme (○) was estimated to be 56 kDa by size-exclusion chromatography on a calibrated Sephacryl HR S 200 column (1.5 cm×85 cm), as described in the Experimental section. Standard proteins (▲) used for calibration were: alcohol dehydrogenase (150000 Da), BSA (67000 Da), chymotrypsinogen A (25000 Da) and cytochrome c (12500 Da), and a calibration curve of log molecular masses of protein markers versus partition coefficients, Kav, was constructed. (C) PDA synthase activity of CG6781 was assayed as described in the Experimental section. The UV–visible spectrum of the reaction product eluted between 21 and 22 min was identical with that of PDA. The characteristic UV–visible spectrum of PDA determined with photodiode array detector (Waters 996) is shown.