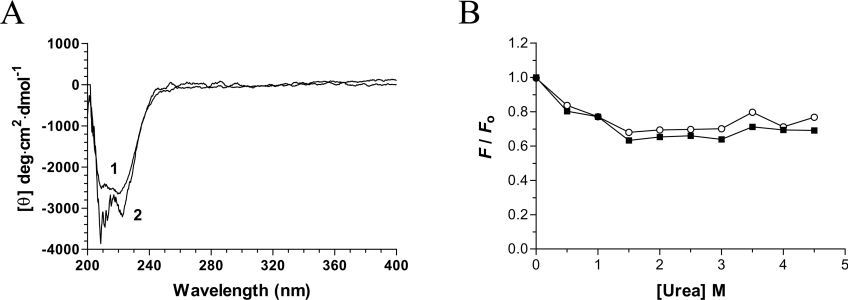
Figure 5. Structural consequences of decavanadate binding.
(A) Far-UV and near-UV CD spectra of the chlorella virus RNA triphosphatase. CD spectra were recorded for the enzyme both in the absence (1) and presence (2) of 2 mM decavanadate. In each case the enzyme concentration was 15 μM and the spectra were recorded from 200 to 400 nm. The average of three wavelength scans is presented. (B) Binding of ANS to the chlorella virus RNA triphosphatase during urea denaturation. The chlorella virus RNA triphosphatase protein was incubated in the absence (■) or presence (○) of 2 mM decavanadate and unfolded with various concentrations of urea at 22 °C for 30 min. Fluorescence emission was monitored after ANS addition (50 μM) at an excitation wavelength of 380 nm. The integrated fluorescence area between 400 and 600 nm was evaluated.