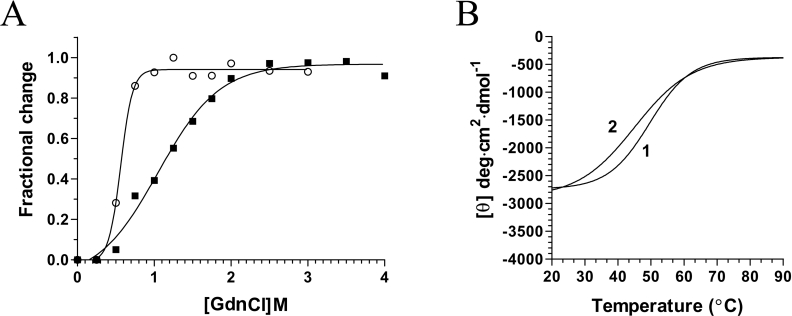
Figure 6. Unfolding equilibrium of the chlorella virus RNA triphosphatase.
(A) Transition curves for GdnCl-induced unfolding of the enzyme were determined. The protein was pre-incubated in the absence (■) or presence of 1 mM decavanadate (○), and denatured with increasing concentrations of GdnCl. Equilibrium unfolding transitions were monitored by integration of the fluorescence intensity. (B) Thermal denaturation of the chlorella virus RNA triphosphatase enzyme. Thermal denaturation was recorded for the unliganded protein (1), or the protein incubated in the presence of 2 mM decavanadate (2). CD spectra were recorded at a constant wavelength of 222 nm from 20 to 90 °C at a protein concentration of 15 μM.