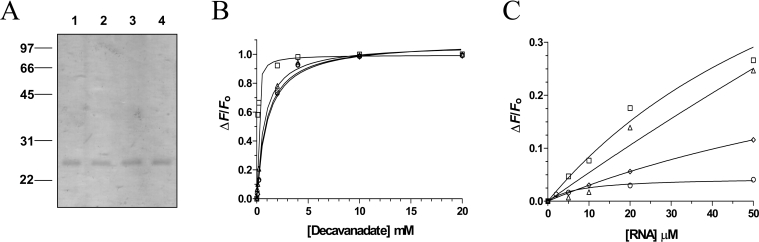
Figure 8. Characterization of the RNA triphosphatase alanine mutants.
(A) The purified proteins were analysed by electrophoresis on 12.5% PAGE gels containing 0.1% SDS and visualized by staining with Coomassie Blue. Aliquots (3 μg) of the 200 mM fraction of the wild-type protein (lane 1), R76A (lane 2), K129A (lane 3) and R131A (lane 4) mutants were analysed. The positions and sizes (in kDa) of the molecular-mass markers are indicated on the left. (B) Fluorescence spectroscopy assays were performed by incubating the wild-type protein (□), R76A (○), K129A (△) and R131A (◇) mutants (480 nM) with increasing amounts of decavanadate. Excitation was performed at 290 nm, and emission was monitored from 310 to 440 nm. The saturation isotherms were generated by plotting the change in the fluorescence intensity at 333 nm as a function of added decavanadate. (C) Fluorescence spectroscopy assays were performed by incubating the wild-type protein (□), R76A (○), K129A (△) and R131A (◇) mutants (480 nM) with increasing amounts of RNA. Excitation was performed at 290 nm, and emission was monitored from 310 to 440 nm. The saturation isotherms were generated by plotting the change in the fluorescence intensity at 333 nm as a function of added RNA.