Figure 2.
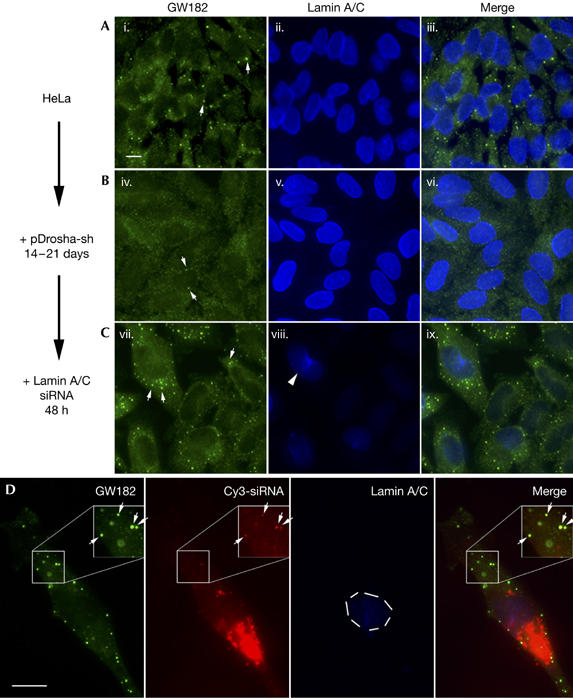
Loss of GW body staining in Drosha-deficient HeLa cells and transfection of synthetic short interference RNAs rescues GW bodies. HeLa cells transfected with pDrosha-sh plasmid were allowed to grow in the presence of 2 μg/ml puromycin to select for cells that were later transfected with lamin A/C short interference RNA (siRNA). (A) Untreated HeLa cells, (B) Drosha-deficient cells selected for 14–21 days, and (C) these cells 48 h after transfection with siRNA were co-stained with GW182 antibody and Alexa 488-goat anti-human IgG (green, i,iv,vii), and lamin A/C monoclonal antibody and Alexa 350-goat anti-mouse IgG (blue, ii,v,viii). GW bodies (GWBs; arrows) were readily detected in untreated HeLa cells (i). Most of the GWB staining was lost in the Drosha-deficient cells, but dispersed residual GWB staining was still apparent (arrows, iv). Many GWBs were observed in Drosha-deficient cells after transfection with siRNA (arrows, vii). The knockdown of lamin A/C in Drosha-deficient cells transfected with lamin A/C siRNA was effective (viii), with only few cells having detectable lamin A/C (arrowhead). (D) Cy3-labelled siRNA for lamin A/C localized to GWBs in transfected Drosha-deficient cells. Most of the Cy3-siRNA seemed to be aggregated in the cytoplasm but some was detected in GWBs (arrows). Efficient lamin A/C knockdown was observed and was indicated by the absence of lamin A/C staining in the nucleus (dashed circle). Insets are enlarged 1.5-fold and the Cy3 signal is enhanced to show the localization of siRNA to GWBs. Scale bars, 10 μm.
