Figure 1.
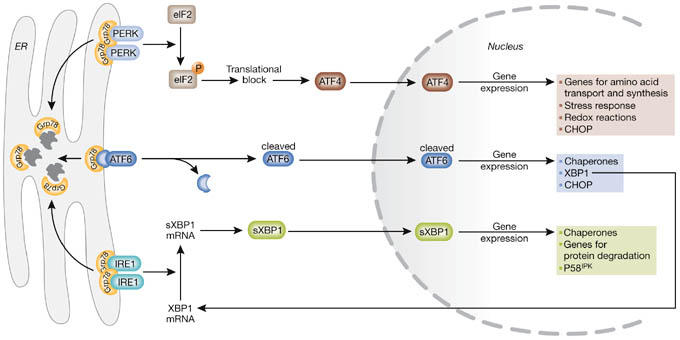
The unfolded protein response. On aggregation of unfolded proteins, GRP78 dissociates from the three endoplasmic reticulum (ER) stress receptors, pancreatic ER kinase (PKR)-like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring enzyme 1 (IRE1), allowing their activation. The activation of the receptors occurs sequentially, with PERK being the first, rapidly followed by ATF6, whereas IRE1 is activated last. Activated PERK blocks general protein synthesis by phosphorylating eukaryotic initiation factor 2α (eIF2α). This phosphorylation enables translation of ATF4, which occurs through an alternative, eIF2α-independent translation pathway. ATF4, being a transcription factor, translocates to the nucleus and induces the transcription of genes required to restore ER homeostasis. ATF6 is activated by limited proteolysis after its translocation from the ER to the Golgi apparatus. Active ATF6 is also a transcription factor and it regulates the expression of ER chaperones and X box-binding protein 1 (XBP1), another transcription factor. To achieve its active form, XBP1 must undergo mRNA splicing, which is carried out by IRE1. Spliced XBP1 protein (sXBP1) translocates to the nucleus and controls the transcription of chaperones, the co-chaperone and PERK-inhibitor P58IPK, as well as genes involved in protein degradation. This concerted action aims to restore ER function by blocking further build-up of client proteins, enhancing the folding capacity and initiating degradation of protein aggregates. CHOP, C/EBP homologous protein.
