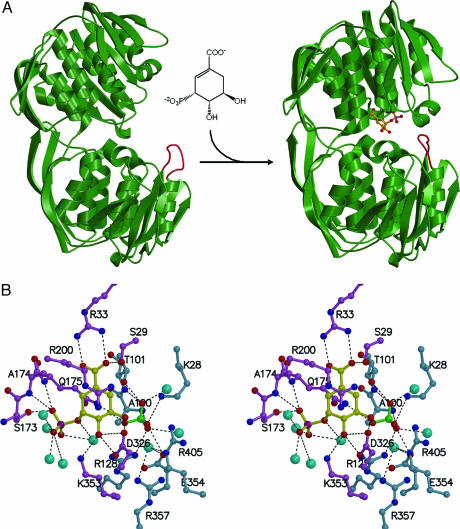
Fig. 2.
Three-dimensional structure of CP4 EPSP synthase. (A) (Left) Unliganded CP4 EPSP synthase exists in an open conformation. (Right) Upon interaction with S3P, the enzyme undergoes a large conformational change to a closed state. Shown in orange is a loop spanning residues 347–358, which is highly flexible in the open conformation but becomes ordered in the closed conformation. This loop contains the strictly conserved EPSP synthase residues Glu-354 and Arg-357, which are involved in PEP/glyphosate binding. Monovalent cations may influence the conformation of this loop and facilitate binding of PEP. (B) Stereoview showing that, in the binary complex, S3P (yellow) binds to the enzyme residues shown in magenta through multiple hydrogen-bonding/electrostatic interactions (black dotted lines). In addition, the cyclohexene moiety of S3P is sandwiched between Arg-200 and Gln-175. Residues shown in light blue constitute the PEP/glyphosate binding site. Attracted by the accumulation of positive charges, a sulfate ion (shown in green) from the crystallization solution binds to the space occupied by the phosphate moiety of PEP or the phosphonate moiety of glyphosate in either ternary complex. Water molecules are shown as cyan spheres.