Abstract
Many infectious viruses coevolved with the vertebrate immune system. During the assembly of enveloped viruses, lipid ordered domains of the host cell plasma membrane, called lipid rafts, frequently function as a natural meeting point for viral proteins. The role of lipid rafts in the organization of complex combinations of immune receptors during antigen presentation and T cell signaling is widely recognized. In our studies, we determined whether lipid rafts, virus budding, and molecular interactions during T cell activation could be brought into a novel context to create artificial antigen-presenting particles. We show here that cell-free virus-like particles (VLP) expressing a surrogate TCR/CD3 ligand (OKT3scFv) and the costimulator CD80 polyclonally activate human T cells independently of accessory cells. VLP expressing the glycoprotein epitope 33–41 of the lymphocytic choriomeningitis virus in the context of H-2Db activate and expand naïve, antigen-specific CD8+ T lymphocytes and differentiate them into cytotoxic effector cells. Efficient targeting of T cell ligands to lipid rafts and ultimately to VLP is achieved by C-terminal introduction of glycosyl phosphatidyl inositol acceptor sequences, replacing transmembrane and intracellular domains. In this work, basic functions of immunostimulatory molecules meet virus biology and translate into a reductionist antigen-specific T lymphocyte-stimulating vehicle, which we refer to as immunosomes. A large variety of agonistic and antagonistic accessory molecules on genuine antigen-presenting cells may complicate the predictable manipulation of T cells as well as the analysis of selected receptor combinations, making immunosomes potentially useful reagents for such purposes in the future.
Keywords: artificial antigen-presenting cells, enveloped viruses, immunomodulation, lipid rafts
T cell activation is the result of a sustained antigen-specific interaction of a T lymphocyte with an antigen-presenting cell (APC) (1) in a secondary lymphatic tissue (2). In the past, protocols for the ex vivo generation and expansion of professional APCs have been established (3). A growing number of activating and inhibitory accessory molecule pairs (cell-bound and soluble) have been identified (4), which makes the net effect of APCs used as cellular stimulants not easy to predict and direct. Another critical point might be the limited availability of APCs, as well as the growing number of described maturation and differentiation stages of dendritic cells (DCs) (5), which makes their use cumbersome, if not impractical. To provide infinite numbers of APCs, xenogenic antigen-presenting systems have been developed in the past (6, 7), imposing, however, the risk of unintended introduction into the human body of microbial sequences along with the transferred cells (8).
Both basic and applied research would profit from a reductionist approach for T lymphocyte activation based on an inducible, natural assembly system for surface proteins that has coevolved with the vertebrate immune system. The requirements are to keep the variables low while creating a fully functional and ideally modular T cell stimulatory unit, which consists of a flexible composition of TCR (T cell receptor) ligands, costimulatory and adhesion molecules, and even cytokines.
Here, we describe immunostimulatory, virus-like particles (VLP), which we refer to as immunosomes, that fully meet the above criteria. The term immunosome was originally used to describe artificial liposomes decorated with viral surface proteins (9). Our definition of immunosomes is broader because it includes inducible plasma membrane-derived particles expressing immunoreceptors of choice. It is well established that enveloped viruses preferentially bud from the lipid raft regions of producer cells (10–12). Lipid rafts are hydrophobic, lipid ordered domains of the plasma membrane, which mainly attract proteins with distinct posttranslational lipid modifications (13). Rafts integrate cellular signaling processes in many cell types including T lymphocytes (14). To make lipid rafts a meeting point for TCR/CD3 ligands and accessory molecules, we genetically modified the molecules of interest with glycosylphosphatidyl inositol (GPI) anchor-acceptor sequences (15) of well described GPI-anchored proteins (e.g., CD14 and CD16). The GPI-modified immunoreceptors become efficiently targeted to lipid rafts (16) and thus to budding VLP.
By mimicking the natural assembly of enveloped viruses, we not only create scaffolds for TCR ligands and accessory molecules relevant for T cell activation, but we also take advantage of the biophysical characteristics of a natural backbone: lipid raft-enriched plasma membranes. They are highly qualified to physically interact with eukaryotic cells to transmit signals (17) or genetic information (18), respectively.
Results
Induction of VLP Production in HEK-293 Cells.
VLP have been shown to consist of a multimerized array of 1,000–1,500 viral core molecules surrounded by a lipid envelope obtained from the host cell plasma membrane (19).
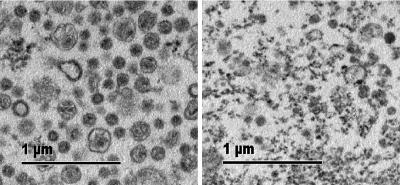
We attempted to determine whether viral core protein expression in the absence of envelope proteins is sufficient to induce particle secretion in HEK-293 cells. Transmission electron microscopy showed spherical plasma membrane-like structures with a diameter of ≈100 nm in supernatants of HEK-293 cells expressing Moloney murine leukemia virus (MoMLV) core proteins (Fig. 1 Left), which supports previous reports (20). The activity of a particle-bound tracer (placental alkaline phosphatase) in the culture supernatant of core protein transfectants was enriched >10-fold over background (data not shown). In contrast, supernatants of control transfectants lacked vesicular structures and contained only cellular debris (Fig. 1 Right).
Fig. 1.
Visualization of HEK-293-derived VLP by transmission electron microscopy. Particulate material in supernatants of HEK-293 cells, which were transfected with MoMLV original gag-pol (Left, +OGP) or control DNA (Right, −OGP), was pelleted by ultracentrifugation and processed for transmission electron microscopy. Shown is one of several similar experiments.
GPI-Modified T Cell Ligands Are Targeted to Lipid Rafts of HEK-293 Cells, Decorate VLP, and Retain Their Functional Activity.
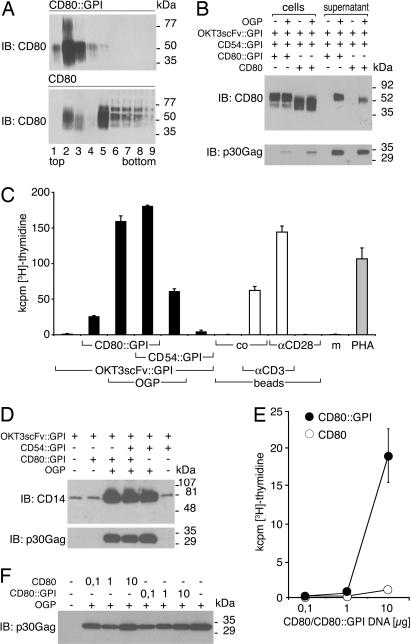
For the delivery of Signal 1 to T lymphocytes, we expressed a single-chain variable fragment (scFv) of the OKT3 hybridoma (recognizing CD3 epsilon) on the cell surface of HEK-293. The use of OKT3scFv as surrogate TCR ligand allowed the optimization of the system with T cells from random donors. For effective targeting to lipid rafts and consequently to VLP, the OKT3scFv was fused to the GPI-anchored molecule CD14 (see Fig. 6, which is published as supporting information on the PNAS web site). Similarly, the costimulatory molecule CD80 and the intercellular adhesion molecule 1 (ICAM-1, CD54) were modified at their C terminus with the GPI anchor-acceptor sequence of the low-affinity Fc gamma receptor III (CD16) (Fig. 6). GPI anchoring was confirmed by substantial release of molecules upon phosphatidyl inositol-specific phospholipase C treatment of transfectants (data not shown). Membrane fractionation experiments of transfectants revealed that CD80::GPI localized exclusively to the lipid raft fractions 2 and 3 (Fig. 2A). Comparable targeting was observed for OKT3scFv::GPI, CD54::GPI (ICAM-1::GPI), and the constitutively GPI-anchored molecule CD59 (data not shown). In contrast, full-length CD80 was mainly targeted to the detergent-soluble fractions of gradients (Fig. 2A).
Fig. 2.
Biochemical and functional characteristics of immunomodulatory VLP. (A) GPI modification targets CD80 to lipid rafts. HEK-293 cells transfected with CD80::GPI or full-length CD80 were lysed at 4°C with 1% Triton X-100 and fractionated on 5–40% sucrose into nine fractions (from top to bottom). Equal amounts of each fraction were resolved by SDS/PAGE, blotted, and probed (immunoblotting) with a CD80-specific mAb. Bound antibody was visualized by goat anti-mouse Ig conjugated to HRP. (B) Preferential targeting of CD80::GPI to VLP. HEK-293 cells were transfected with the indicated cDNAs or control plasmid, and cell lysates, as well as corresponding supernatants, were analyzed by SDS/PAGE followed by immunoblotting with a CD80 mAb. As a control for proper vesicle induction and loading, viral core protein (p30 Gag) expression was determined. (C) Stimulation of human T cells by VLP. Human PBMC (105) were cocultured with supernatants of stably OKT3scFv::GPI-expressing HEK-293 that were cotransfected with OGP, CD80::GPI, CD54::GPI, or control plasmid alone, or combined as indicated (black bars). Microbeads (105 per well), substituted with CD3 mAb, CD28 mAb, control mAb (co), or combinations thereof, were used for comparison (unshaded bars). PHA or culture medium (m) served as controls (gray bars). Cell proliferation was determined after 4 days by [3H]thymidine uptake. Data show mean values +SD of triplicate cultures representative of several independently performed experiments. (D) The amount of applied particles in proliferation assays (used in C) was determined by immunoblotting for OKT3scFv::GPI by using a CD14 mAb, and for viral core proteins by using a p30 Gag mAb. (E) The membrane anchor of CD80 determines the stimulatory capacity of immunosomes. Full-length CD80 or CD80::GPI was cotransfected in titrated amounts into OKT3scFv::GPI-expressing HEK-293 cells along with gag-pol. Immunosome-containing supernatants were tested for their T cell-activating potential by [3H]thymidine uptake after 4 days of culture. Data show mean values of three experiments ± SD. (F) To control for homogeneous particle production, individual supernatants used for stimulation in E were probed with a MoMLV Gag-specific mAb.
Are the Lipid Raft-Targeted T Cell Ligands Decorating VLP, and if So, Do They Remain Functionally Active?
Fig. 2B shows viral core protein-dependent targeting of CD80::GPI to VLP (see Fig. 7, which is published as supporting information on the PNAS web site). Similar results were obtained for OKT3scFv::GPI and CD54::GPI (data not shown). In contrast, full-length CD80 was targeted with lower efficiency although amounts of secreted particles were comparable, as shown by immunoblotting for p30 Gag (Fig. 2B). Significantly, no substantial spontaneous CD80 export was observed in the absence of viral core protein expression. In functional terms, supernatants of HEK-293 cells expressing OKT3scFv::GPI were unable to induce proliferation of peripheral blood mononuclear cells (PBMC) (Fig. 2C). In the absence of enhanced particle formation, coexpression of CD80::GPI, but not of CD54::GPI (ICAM-1::GPI), led to moderate proliferative responses. However, coexpression of viral core proteins (original gag-pol, OGP) augmented lymphocyte proliferation induced by cellular supernatants >5-fold, which was also mirrored by the clear-cut increase in the amount of secreted particles as shown by immunoblotting for OKT3scFv::GPI (CD14) and viral core protein p30 Gag (Fig. 2D). Under similar conditions, CD54::GPI (ICAM-1::GPI) synergized with signal one to a much lesser extent than CD80::GPI, although similar amounts of particles were applied (Fig. 2D). Synergy between coexpressed CD54::GPI (ICAM-1::GPI) and CD80::GPI could only be observed when CD80::GPI expression was suboptimal (data not shown). Control experiments showed that the T lymphocyte-activating potency of OKT3scFv::GPI plus CD80::GPI-decorated VLP is comparable with, if not superior to, microbeads substituted with CD3 and/or CD28 mAbs or phytohemagglutinin (PHA) (Fig. 2C).
Fig. 2E shows that significant immunogenicity of VLP was achieved only when CD80::GPI, but not full-length CD80, was expressed in producer cells, although similar amounts of VLP were applied (Fig. 2F).
At this point, we propose that immunostimulatory VLP be called immunosomes. Originally, the term immunosome was coined to describe liposomes coated exclusively with viral surface glycoproteins (9). We think, however, that VLP decorated with immunomodulatory ligands are also best described by this name.
Purification and Quantification of Immunosomes.
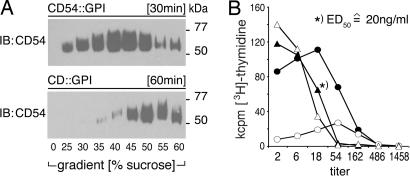
In our initial experiments, we used fresh, crude cell-culture supernatants (0.2 μm filtered) as a source of immunosomes. Subsequently, ultracentrifugation was identified as an effective method for the concentration and quantitative recovery of immunosomes. Fig. 3A shows that all of the CD54::GPI, used as tracer in sedimentation assays, can be pelleted and is thus particle-bound. By comparing the functional activities of titrated amounts of crude supernatants with that of purified preparations, we were able to calculate the amount of immunosomes also in crude supernatants (Fig. 3B). We observed that 2 × 106 HEK-293 cells produce ≈1 μg of immunosomes within 24 h. The experiments shown in Fig. 2 C and E were performed with saturating concentrations of immunosomes (500 ng/ml). However, half maximal T cell proliferation (ED50) could be achieved with immunosome concentrations as low as 20 ng/ml. Immunosomes were stable at −80°C; however, there was ≈50% loss because of ultracentrifugation.
Fig. 3.
Concentration and physical stability of immunosomes. (A) Time-dependent sedimentation of immunosomes by ultracentrifugation. Immunosomes expressing OKT3scFv::GPI, CD80::GPI, and CD54::GPI were loaded onto 25–60% sucrose gradients and ultracentrifuged for 30 or 60 min. Sucrose (0%) represents the loaded cell culture supernatant fraction. Centrifugation was stopped at indicated time points. The fractions were collected and subjected to SDS/PAGE followed by immunoblotting with an ICAM-1 (CD54)-specific mAb. (B) Concentration and storage of immunosomes. The functional capability of the above immunosomes (expressing OKT3scFv::GPI, CD80::GPI, and CD54::GPI) was determined before and after purification and/or storage at −80°C. For that purpose, PBMC (105) were incubated with titrated amounts of crude (filled circles) or cleared (open circles) supernatant, purified immunosomes (filled triangles), or purified immunosomes stored at −80°C (open triangles) adjusted to the original volume. Proliferation was determined after 4 days by [3H]thymidine uptake. Determination of protein concentration of immunosomes was performed according to standard procedures (concentration indicated for ED50). Data show mean values of one representative experiment of two.
Immunosomes Activate T Cells in the Absence of Accessory Cells.
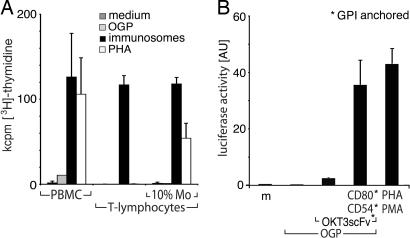
Exosomes are immunostimulatory microvesicles spontaneously secreted by, e.g., DC and Epstein–Barr virus B blasts (21, 22), which require cross-presentation by DC to activate T lymphocytes (21). In contrast, incubation of highly purified (>99%) human PB T cells with immunosomes expressing OKT3scFv::GPI, CD80::GPI, and CD54::GPI revealed a strong proliferative response, which was comparable to that of PBMC or purified T cells supplemented with 10% monocytes (Fig. 4A). In addition, coincubation of Jurkat T cells, expressing an IL2-luciferase reporter gene, with Signal 1 and 2 expressing immunosomes, led to strong cellular activation (Fig. 4B), comparable with the positive control PHA/phorbol 12-myristate 13-acetate (PMA). Significantly, both cellular systems were strictly dependent on costimulation.
Fig. 4.
Accessory cell-independent activation of T lymphocytes by immunosomes. (A) PBMC, highly purified T cells, or T cells supplemented with 10% monocytes were stimulated with medium, undecorated VLP induced by gag-pol (OGP), immunosomes (expressing OKT3scFv::GPI, CD80::GPI, and CD54::GPI), or PHA. Cellular proliferation was determined after 4 days by [3H]thymidine uptake. Data show mean values of two experiments +SD. (B) Jurkat T cells expressing luciferase under the IL-2 promoter/enhancer were stimulated with medium, undecorated VLP, immunosomes expressing the indicated constructs, or PHA plus PMA. Luciferase activity was determined in cell lysates after a cocultivation period of 5 h. Data show mean values (arbitrary units) of three independent experiments +SD.
The affinity of the surrogate TCR/CD3 ligand that we used is most likely different from classical medium- to low-affinity MHC/peptide ligands. Nevertheless, the surrogate TCR/CD3 ligand helped to establish the system and might prove valuable for studying human immunomodulatory molecules in a relevant membrane context in the future.
Immunosomes Activate Antigen-Specific T Lymphocytes and Generate Potent Effector Functions.
Finally, we sought to clarify whether immunosomes are also able to induce antigen-specific immune responses. For that purpose, we replaced the above-described surrogate TCR/CD3 ligand by a bona fide restriction element, i.e., the murine H-2Db molecule in GPI-anchored form. H-2Db::GPI was expressed along with murine β2-microglobulin, murine CD54::GPI (ICAM-1::GPI), murine CD80::GPI, and a peptide minigene coding for the immunodominant CD8+ T cell epitope of lymphocytic choriomeningitis virus glycoprotein peptide 33–41 (LCMV-GP33–41).
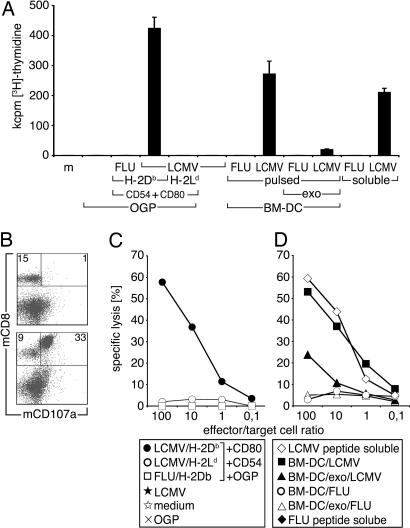
Splenocytes from transgenic mice expressing the P14-TCR specific for LCMV-GP33–41 (23) were cocultured with purified LCMV-specific immunosomes expressing LCMV-GP33–41 in the context of H-2Db::GPI. As controls, purified immunosomes expressing the LCMV-GP33–41 in the context of H-2Ld::GPI, an influenza peptide (FLU-MA58–66) in the context of H-2Db::GPI, or undecorated immunosomes were used. A specific primary immune response, as measured by cellular proliferation, was observed only when the LCMV peptide was coexpressed in the context of H-2Db::GPI but not in any of the other immunosome combinations tested (Fig. 5A). Within 4 days, we observed a >15-fold increase of CD8+ T cell numbers (data not shown). The degree of splenocyte proliferation observed upon coculture with LCMV-specific immunosomes was comparable with, if not superior to, the proliferation observed upon coculture with bone marrow-derived (BM) DC pulsed with 10−9 M of LCMV peptide or soluble LCMV peptide (10−9 M) directly added to the splenocytes (Fig. 5A). No proliferation was inducible with the corresponding LCMV-specific exosomes of such BM-DC (data not shown). However, when combined with nonpulsed BM-DC, weak but consistent cellular proliferation could be detected. In addition, exosomes of LCMV-GP-pulsed, syngeneic EL4 cells also led to moderate T cell proliferation (≈5 × 104 cpm) when combined with BM-DC (data not shown). No proliferation was detectable by pulsing BM-DC with FLU M158–66 peptide or by adding it directly to the culture (Fig. 5A).
Fig. 5.
Antigen-specific T lymphocyte activation by LCMV GP33–41-specific immunosomes. (A) Spleen cells (2 × 105) from P14 TCR transgenic mice were cocultured with 2.5 μg/ml of LCMV GP33–41/H-2Db::GPI-expressing immunosomes, as well as with control immunosomes or undecorated VLP. All indicated membrane molecules on immunosomes were GPI-anchored. BM-DC (2 × 104) pulsed with LCMV GP33–41 or FLU M158–66 peptide (10−9 M), as well as corresponding exosome preparations (2.5 μg/ml) and combinations thereof, as well as soluble peptides added directly to splenocytes (10−9 M) were used for comparison. Cell proliferation was determined after 4 days by [3H]thymidine uptake. Data show mean values of triplicate cultures +SD, which are representative of several independently performed experiments. (B) Flow cytometric analysis of spleen cells after 48 h cocultivation with control (Upper) or LCMV GP33–41-specific (Lower) immunosomes. T lymphocytes were stained with directly conjugated CD8 mAb and CD107a (LAMP1) mAb, a marker for degranulating CTLs. Numbers indicate percent positive cells in respective quadrants. The data show one representative experiment of several performed. (C) The lytic activities of spleen cells cocultured with LCMV-specific or -nonspecific immunsomes or undecorated VLP were determined after 4 days of culture. (D) Control experiments with spleen cells cocultured with BM-DC pulsed with LCMV GP33–41 or FLU M158–66 peptide, corresponding exosomes combined with nonpulsed BM-DC, or soluble peptides are shown. EL4 cells pulsed with LCMV GP33–41 served as targets in 5 h 51Cr-release assays. Data are representative for four experiments performed.
Are the T Lymphocytes Activated by Immunosomes Classical Effector Cells?
Upon coculture of P14 splenocytes with antigen-specific immunosomes, surface CD107a (LAMP-1) expression, which is indicative of cytotoxic granule exocytosis (24), was already detected on the major subset of CD8+ T cells after 2 days (Fig. 5B), peaking on day 4 (data not shown). Additionally, the cell population expanded in the presence of LCMV-specific immunosomes was cytotoxic and lysed syngeneic EL4 target cells that were pulsed with LCMV-GP33–41 (Fig. 5C) with high efficiency but not EL4 cells pulsed with FLU-MA58–66 peptide or native EL4 cells (data not shown). In contrast, splenocytes cultured in the presence of the indicated control immunosomes (see above) were neither activated nor did they become effector cells. The cytotoxic potential of cytotoxic T lymphocytes (CTL) activated in the presence of LCMV-specific immunosomes was comparable with CTL activated in the presence of LCMV-GP33–41-pulsed BM-DC or with splenocytes, which were directly incubated with 10−9 M of soluble LCMV-GP33–41 peptide (Fig. 5D). When LCMV-specific exosomes were combined with BM-DC, they induced detectable but much weaker CTL activity. In addition to stimulating TCR Tg lymphocytes, LCMV-specific immunosomes activated antigen-specific CTL from spleens of wild-type C57BL/6 mice, with an estimated precursor frequency of ≈1 in 3 × 105 CD8+ T cells (data not shown).
Discussion
Inducible VLP, decorated with T cell ligands of choice, can be directly immunogenic for purified T lymphocytes. We found that lipid rafts are the keys to inducible VLP production and the selective enrichment of immunologically relevant molecules on VLP. VLP-expressing combinations of T cell ligands plus antigen were produced and their impact on T cell activation was evaluated. Functional analyses demonstrated that they mediate strong, accessory cell-independent, antigen-specific T lymphocyte activation.
In our system, TCR/CD3 ligation or delivery of secondary signals alone is insufficient to activate human T cells. However, when used in combination, strong activation according to the two-signal model can be observed. Additional synergy can be achieved in the system by the adhesion molecule CD54::GPI (ICAM-1::GPI) or by membrane bound cytokines (data not shown), although only when stimulation by TCR ligand and CD80::GPI is suboptimal. Experiments with antigen-specific immunosomes show that “internal loading” of MHC class I molecules, in our case via coexpressed peptide minigenes, is possible and that the resulting peptide binding is stable enough to withstand prolonged storage or even freeze–thaw cycles (data not shown). This offers the attractive possibility to also introduce complex antigens or even antigen mixtures into producer cells and thereby select for high-affinity peptides.
On a biochemical level, we demonstrated that lipid modification targets all of the T cell ligands tested so far to lipid rafts of the producer cells and, consequently, to immunosomes. GPI anchor-acceptor sequences of CD14, CD16, CD55, and CD59 were found to be comparably effective (data not shown). Notably, similar sorting accuracy has previously been observed with heterologous envelope proteins during viral pseudotyping (10). Although GPI-modified T cell ligands are targeted to lipid rafts, they do not induce vesicle secretion on their own. Although several aspects of the viral budding process remain unresolved (20, 25), it was clearly demonstrated that specialized core proteins, which attach themselves to the inner layer of lipid rafts, play a key role (26). In this study, we took advantage of the group-specific antigens (Gag) of Moloney murine leukemia virus (MoMLV) as the vesicle-inducing principle. However, core proteins of other enveloped viruses unrelated to Retroviridae might be used for this purpose as well, with comparable success in the future (27, 28).
Taken together, our data illustrate that immunosomes are quite dissimilar to exosomes. First, immunosomes originate, per definition and according to their virus-like nature, from the cellular plasma membrane (20) and are highly inducible by expression of viral core proteins. In contrast, exosomes are reported to result from inward budding of endo/lysosomal membranes, which are released by the cell (29). Nevertheless, recent reports argued for a distinct overlap between exosome generation and retroviral budding (30). Second, immunosomes are able to stimulate T cells directly whereas exosomes must be cross-presented by DC (31) to activate T cells. Third, the molecular composition of immunosomes can be designed easily at the level of transfection of HEK-293, a cell line, which proved to be highly useful for the production of other biologicals in the past. In contrast, the process of exosome secretion, e.g., from DC, occurs in a constitutive fashion with the molecular composition and especially the donor-dependent MHC allotypes being not easily modifiable (21).
Our data demonstrate virus biology, lipid rafts, and aspects of lymphocyte activation in a novel biological context. Although not formally addressed in this study, strong evidence exists that viral incorporation of immunoregulatory molecules is of biological relevance during infection. For example, HIV is able to coat itself with host cell-derived, GPI-anchored, complement regulatory proteins (e.g., decay-accelerating factor, CD55) and thereby protects itself from inactivation by human complement (32). Varmus and coworkers (33) showed that even a coreceptor for T lymphocyte activation, i.e., CD4, can become efficiently incorporated into retroviruses, a phenomenon subsequently also shown for DNA viruses (34). Taken together, our approach of intentionally decorating VLP with immunomodulatory molecules is not completely unprecedented by nature and may even occur regularly. Whether viruses also use their natural coating with immunomodulatory molecules to induce cellular activation, as described for immunosomes, remains to be shown.
Our findings may also have practical implications. VLP may serve as novel, modular, lipid raft-based, artificial antigen-presenting platforms, or even as multivalent binding reagents. They will complement already existing artificial antigen-presenting systems such as aAPC (6) and exosomes (29). In related applications, immunosomes could potentially be equipped with inhibitory receptors or even cytokines to shape (activate/tolerize) T lymphocyte responses. In addition, induction of B cell immunity may become simplified by our pseudotyping approach. Natural adjuvants (immunomodulators) could possibly be introduced into vaccine strains of enveloped viruses without needing to alter the viral genome itself.
Materials and Methods
Cell Lines and Primary Cells.
HEK-293 and EL4 cells (American Type Culture Collection, Manassas, VA), and Jurkat clone 41-19 supplemented with 0.8 μg/ml G418 (Invitrogen, Carlsbad, CA) were maintained in IMDM medium (PAA, Pasching, Austria) as described (10). Peripheral blood was collected with informed consent. PBMC and purified T cells (>99% by flow cytometry) were obtained according to standard procedures.
Plasmid Constructs and Transfections.
The ectodomains of T cell ligands were PCR-amplified from cDNA clones (hCD54), cDNA libraries (hCD80), or freshly prepared cDNA obtained from C57BL/6 (H-2Db, mCD54, mCD80, mβ2m) or BALB/c (H-2Ld) splenocytes, and were inserted downstream of a human CD5 leader cassette into the pEAK12 vector (Edge Biosystems, Gaithersburg, MD). For GPI anchoring, transmembrane and intracellular domains were replaced by the CD16 GPI anchor-acceptor sequence (Fig. 6). MoMLV original gag-pol (OGP) was expressed from pMD gag-pol vector kindly provided by R. Mulligan (Children’s Hospital, Boston, MA). OKT3scFv was constructed as described (35), fused to CD14, and expressed in pEAK12 (Fig. 6). HEK-293 OKT3scFv stables were obtained by transfection of linearized plasmid and selection with 1 μg/ml puromycin. The LCMV-GP33–41 (23) and FLU M158–66 (36) minigenes were generated by inserting synthetic oligonucleotides with NheI- and NotI-compatible ends into pEAK12 downstream of the CD5 signal sequence.
Immunosome Production.
HEK-293 cells (3 × 106), which have successfully been used to produce other biologicals in the past, were transfected with plasmid DNA (30 μg per 10-cm Petri dish) by Ca2PO4-transfection. Where indicated, 7.5 μg of OGP was included to induce VLP production. If necessary, DNA amounts were brought to 30 μg with empty pEAK12 vector. The medium was changed 18 h after transfection, and supernatants were harvested 48 h later (Fig. 7). Cellular debris was removed by centrifugation (900 × g for 10 min) and filtration (0.45 or 0.22 μm; Millipore, Billerica, MA). Supernatants were used immediately (100 μl per well) or, alternatively, particulate material was concentrated and washed in a large volume of PBS by ultracentrifugation (100,000 × g for 1 h), resuspended in PBS, and used immediately or frozen at −80°C. Protein concentration of VLP was determined by standard procedures (BCA Kit; Pierce, Rockford, IL).
Biochemical Procedures.
Whole-cell lysates and lipid rafts were prepared as described (10). Particulate material in supernatants (immunosomes) was concentrated as described above and resuspended in 300 μl of sample buffer. Sucrose gradients (0.5 ml, 25–60%) were overlaid with 8 ml of fresh supernatant and centrifuged (100,000 × g for 30 or 60 min). Fractions were subjected to SDS/PAGE (10%). Proteins were transferred to PVDF membranes (Millipore) and subjected to immunoblotting. CD80 mAb MEM-233 (Exbio, Prague, Czech Republic), CD54 mAb MEM-111 (provided by V. Horejsi, Institute of Molecular Genetics, Prague, Czech Republic), MoMLV p30 Gag mAb R187 (American Type Culture Collection), and CD14 antiserum AB383 (R & D Systems, Minneapolis, MN) were used at 1 μg/ml. HRP-conjugated secondary antisera (DAKO, Glostrup, Denmark) were used at a dilution of 1:104.
Electron Microscopy.
Supernatants of native or OGP-transfected cells were pelleted and washed by ultracentrifugation as described above. Pellets were fixed (2% glutardialdehyde/PBS, 2 h, 22°C), postfixed (1% OsO4 PBS), and dehydrated and embedded (glycid ether 100) according to standard procedures. Ultrathin sections were stained with uranyl acetate/lead citrate and examined (JEOL 1200 EX II, Tokyo, Japan).
Proliferation Assays.
Cells were cultured in RPMI medium 1640 plus 10% FCS, 2 mM l-glutamine, essential amino acids, 0.1 mM 2-mercaptoethanol, 1 mM sodium pyruvate, and 25 mM Hepes (pH 7.3). PBMC (105) and P14 TCR Tg murine splenocytes (2 × 105) or highly purified T cells (5 × 104) were incubated with 50–100 μl of the indicated immunosome preparations (sups, particulate fraction 2.5 μg/ml), controls (PHA 5 μg/ml or medium), BM-DC (2 × 104), exosomes (2.5 μg/ml), or soluble peptides (10−9 M) in 96-well plates in triplicates (final volume, 200 μl). CD3 mAb OKT3 (eBioscience, San Diego, CA) and/or CD28 mAb 28.2 (BD Pharmingen, San Jose, CA) and/or control mAb VIAP were coated onto 4.5-μm goat anti-mouse IgG magnetic beads (Dynabeads; Dynal Biotech, Oslo, Norway) at a concentration of 150 fg per bead and were used at a bead-to-cell ratio of 1:1. Dendritic cells and exosomes were prepared as described (21). Briefly, BM cells (C57BL/6 mice) were cultured in the presence of IL-4, GM-CSF, and TNF-α (100 units/ml each). The floating cells were transferred on day 5, supplemented with fresh cytokines, and cultured for 2 more days. Subsequently, BM-DC were incubated with LCMV GP33–41 peptide or FLU M158–66 (10−9 M) overnight. BM-DC supernatants were harvested and centrifuged at 300 × g and then 10,000 × g to remove debris. Exosomes were pelleted by centrifugation at 100,000 × g for 1 h and washed once in a large volume of PBS. Protein concentration was determined with the micro BCA test (Pierce, Rockford, IL). After spin inoculation at 900 × g for 1 h and incubation in a humidified atmosphere at 37°C for 48–72 h, individual wells were pulsed with methyl-[3H]thymidine at 1 μCi per well (1 Ci = 37 GBq) for 18 h before harvest (Packard, Meriden, CT).
Jurkat Luciferase Assay.
Jurkat cells (5 × 104) stably transfected with an IL-2-luciferase reporter were plated together with 100 μl of the respective supernatant or control in a final volume of 200 μl in duplicate. After spin inoculation and incubation of plates at 37°C in a humidified atmosphere for 5 h, cells were lysed and luciferase activity was determined (Promega, Madison, WI) on a luminometer (Labsystems, Helsinki, Finland).
Mice.
C57BL/6 mice were obtained from Charles River Laboratories (Sulzfeld, Germany). P14 TCR Tg mice expressing the Vα2/Vβ8 TCR specific for amino acids 33–41 of the LCMV glycoprotein in association with the H-2Db molecule were described previously (23) and were kindly provided by R. Zinkernagel (Institute of Experimental Immunology, Zurich, Switzerland). Animals were kept under conventional conditions and were used for experiments at 8–16 weeks of age.
Cytotoxicity Assay.
P14 splenocytes (2.5 × 106) were cocultured for 4 days with immunosomes (2.5 μg/ml), exosomes (2.5 μg/ml), and dendritic cells (2.5 × 105) pulsed with peptide (LCMV GP33–41 or FLU M158–66, ProImmune, Cambridge, U.K.) or supplemented with exosomes (2.5 μg/ml), or alternatively with soluble peptides (10−9 M). Each day, aliquots of cells were removed from replica wells and stained with CD107a (LAMP1), CD3, CD8 or control mAbs (Becton Dickinson, Franklin Lakes, NJ) to determine cytotoxic vesicle secretion. The cytolytic activity of T cells was tested in standard 51Cr-release assays. EL4 cells (2 × 106/100 μl) were pulsed with LCMV GP33–41 peptide or FLU M158–66 control peptide (10−9 M, overnight) and labeled with 100 μCi of Na51CrO4 (PerkinElmer, Boston, MA) in 200 μl of medium at 37°C for 1 h. After four washes, EL4 cells (104) were added to 96-well plates that contained titrated numbers of prestimulated (see above) spleen cells. All experiments were performed in triplicates. After 5 h of incubation at 37°C, the supernatants were collected and the radioactivity was determined in a γ-counter (Packard). The percentage of specific release was determined as follows: [CTL-induced release (cpm) − spontaneous release (cpm)]/[maximum release (cpm) − spontaneous release (cpm)] × 100.
Supplementary Material
Acknowledgments
Prof. Walter Knapp passed away on August 30, 2004. We will always remember his enthusiasm for science and his mentorship. We are grateful to Drs. R. Zinkernagel for providing P14 Tg mice; V. Horejsi for providing mAbs; and M. Epstein, H. Ploegh, P. Steinberger, and G. Zlabinger for fruitful discussions. This work was supported by Austrian Science Foundation Grants P-15534 and SFB-F1816, Austrian Academy of Sciences Center of Molecular Medicine Grant 20030, and grants from the National Institutes of Health.
Abbreviations
- APC
antigen-presenting cell
- BM
bone marrow-derived
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- FLU
influenza
- GPI
glycosyl phosphatidyl inositol
- ICAM-1
intercellular adhesion molecule 1
- LCMV
lymphocytic choriomeningitis virus
- MoMLV
Moloney murine leukemia virus
- OGP
original gag-pol of MoMLV
- PBMC
peripheral blood mononuclear cells
- scFv
single-chain variable fragment
- TCR
T cell receptor
- VLP
virus-like particle.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Banchereau J., Steinman R. M. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Kundig T. M., Bachmann M. F., DiPaolo C., Simard J. J., Battegay M., Lother H., Gessner A., Kuhlcke K., Ohashi P. S., Hengartner H., et al. Science. 1995;268:1343–1347. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 3.Robinson S. P., Stagg A. J. Dendritic Cell Protocols. Totowa, NJ: Humana; 2001. [Google Scholar]
- 4.Montoya M. C., Sancho D., Vicente-Manzanares M., Sanchez-Madrid F. Immunol. Rev. 2002;186:68–82. doi: 10.1034/j.1600-065x.2002.18607.x. [DOI] [PubMed] [Google Scholar]
- 5.Shortman K., Liu Y. J. Nat. Rev. Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 6.Latouche J. B., Sadelain M. Nat. Biotechnol. 2000;18:405–409. doi: 10.1038/74455. [DOI] [PubMed] [Google Scholar]
- 7.Hwang I., Shen X., Sprent J. Proc. Natl. Acad. Sci. USA. 2003;100:6670–6675. doi: 10.1073/pnas.1131852100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin U., Kiessig V., Blusch J. H., Haverich A., von der Helm K., Herden T., Steinhoff G. Lancet. 1998;352:692–694. doi: 10.1016/S0140-6736(98)07144-X. [DOI] [PubMed] [Google Scholar]
- 9.Perrin P., Sureau P., Thibodeau L. Dev. Biol. Stand. 1985;60:483–491. [PubMed] [Google Scholar]
- 10.Pickl W. F., Pimentel-Muinos F. X., Seed B. J. Virol. 2001;75:7175–7183. doi: 10.1128/JVI.75.15.7175-7183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheiffele P., Rietveld A., Wilk T., Simons K. J. Biol. Chem. 1999;274:2038–2044. doi: 10.1074/jbc.274.4.2038. [DOI] [PubMed] [Google Scholar]
- 12.Bryant M., Ratner L. Proc. Natl. Acad. Sci. USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melkonian K. A., Ostermeyer A. G., Chen J. Z., Roth M. G., Brown D. A. J. Biol. Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 14.Simons K., Toomre D. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 15.Berger J., Howard A. D., Brink L., Gerber L., Hauber J., Cullen B. R., Udenfriend S. J. Biol. Chem. 1988;263:10016–10021. [PubMed] [Google Scholar]
- 16.Brown D. A., Rose J. K. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia A., Sallusto F. Nat. Immunol. 2001;2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 18.Popik W., Alce T. M., Au W. C. J. Virol. 2002;76:4709–4722. doi: 10.1128/JVI.76.10.4709-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layne S. P., Merges M. J., Dembo M., Spouge J. L., Conley S. R., Moore J. P., Raina J. L., Renz H., Gelderblom H. R., Nara P. L. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 20.Coffin J. M., Hughes S. H., Varmus H. E. Retroviruses. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. [PubMed] [Google Scholar]
- 21.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 22.Raposo G., Nijman H. W., Stoorvogel W., Liejendekker R., Harding C. V., Melief C. J., Geuze H. J. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pircher H., Burki K., Lang R., Hengartner H., Zinkernagel R. M. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 24.Betts M. R., Brenchley J. M., Price D. A., De Rosa S. C., Douek D. C., Roederer M., Koup R. A. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 25.Takimoto T., Portner A. Virus Res. 2004;106:133–145. doi: 10.1016/j.virusres.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Gottlinger H. G., Sodroski J. G., Haseltine W. A. Proc. Natl. Acad. Sci. USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Puertas P., Albo C., Perez-Pastrana E., Vivo A., Portela A. J. Virol. 2000;74:11538–11547. doi: 10.1128/jvi.74.24.11538-11547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Justice P. A., Sun W., Li Y., Ye Z., Grigera P. R., Wagner R. R. J. Virol. 1995;69:3156–3160. doi: 10.1128/jvi.69.5.3156-3160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thery C., Zitvogel L., Amigorena S. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 30.Gould S. J., Booth A. M., Hildreth J. E. Proc. Natl. Acad. Sci. USA. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thery C., Duban L., Segura E., Veron P., Lantz O., Amigorena S. Nat. Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 32.Marschang P., Sodroski J., Wurzner R., Dierich M. P. Eur. J. Immunol. 1995;25:285–290. doi: 10.1002/eji.1830250147. [DOI] [PubMed] [Google Scholar]
- 33.Young J. A., Bates P., Willert K., Varmus H. E. Science. 1990;250:1421–1423. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]
- 34.Dolter K. E., King S. R., Holland T. C. J. Virol. 1993;67:189–195. doi: 10.1128/jvi.67.1.189-195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfistershammer K., Klauser C., Pickl W. F., Stöckl J., Leitner J., Zlabinger G., Majdic O., Steinberger P. Eur. J. Immunol. 2006;36:1–10. doi: 10.1002/eji.200535344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gotch F., Rothbard J., Howland K., Townsend A., McMichael A. Nature. 1987;326:881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.