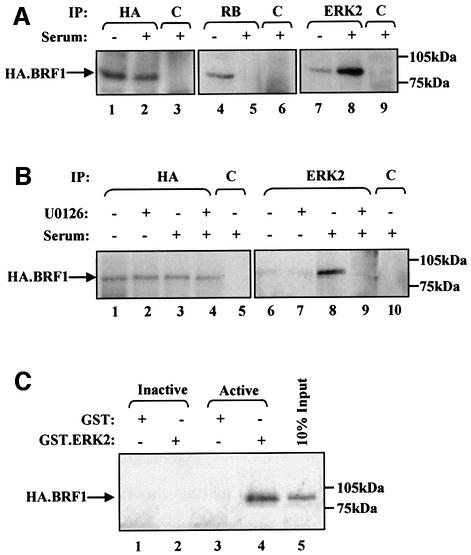
Fig. 4. Activated ERK2 is co-immunoprecipitated with TFIIIB both in vitro and in vivo. (A) Cell extract (500 µg) was prepared from Rat1A fibroblasts expressing pCDNA3HA.BRF1 either cultured for 24 h in serum-free conditions (lanes 1, 4 and 7) or left growing in 5% serum (lanes 2, 3, 5, 6, 8 and 9). These were immunoprecipitated (IP) with anti-HA (lanes 1 and 2), anti-RB antibody C-15 (lanes 4 and 5), anti-ERK2 antibody (lanes 7 and 8) or anti-TAFI48 antibody M-19 (lanes 3, 6 and 9). Precipitates were resolved by SDS–PAGE and then analysed by western blotting with anti-HA antibody. (B) Rat1A fibroblasts expressing pCDNA3HA.BRF1 (500 µg) were cultured for 24 h in serum-free conditions (lanes 1, 2, 6 and 7) or left growing in 5% serum (lanes 3–5 and 8–10) and in the presence (lanes 2, 4, 7 and 9) or absence (lanes 1, 3, 5, 6, 8 and 10) of 1 µM U0126 for a further 2 h. Precipitates were resolved by SDS–PAGE and then blotted with anti-HA antibody. (C) Cell extract prepared from growing Rat 1A cells expressing pCDNA3HA.BRF1 was heat treated at 65°C for 30 min to inactivate endogenous ERK2. Then, 250 µg of this extract was incubated in the presence of glutathione beads carrying equal amounts of GST (lanes 1 and 3) or GST-ERK2 that was left inactive (lane 2) or was activated by MEK (lane 4). Proteins retained after extensive washing were resolved by SDS–PAGE and immunoblotted with anti-HA antibody to detect binding of HA.BRF1.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.