Abstract
The pteroid bone is a rod-like element found only in pterosaurs, the flying reptiles of the Mesozoic. It articulated at the wrist, and supported a membranous forewing in front of the inner part of the wing spar. The function of this bone, particularly its orientation, has been much debated. It is widely believed that it pointed towards the body, and that the forewing was relatively narrow. An alternative hypothesis states that it was directed forwards during flight, resulting in a much broader forewing that acted as a leading edge flap. We tested scale models in a wind tunnel to determine the aerodynamic consequences of these conflicting hypotheses, and found that performance is greatly improved if the pteroid is directed forwards: the lift : drag ratios are superior and the maximum lift is exceptionally high in comparison with conventional aerofoils. This high lift capability may have enabled even the largest pterosaurs to take off and land without difficulty.
Keywords: pterosaur flight, pteroid, propatagium, aerodynamics, functional morphology
1. Introduction
Pterosaurs were the first vertebrates to achieve true flapping flight. They appear in the fossil record at the end of the Triassic period (220 million years ago), and became extinct at the end of the Cretaceous period (65 million years ago), leaving no descendants. Later forms grew to gigantic size with, in some cases, wingspans of 10 m or more. Pterosaur wings were membranous, each consisting of a sail-like cheiropatagium (the main wing membrane) stretched between the fore- and hindlimbs, a crescent-shaped cruropatagium along the medial border of the leg and a propatagium (forewing) in front of the arm. The distal part of the cheiropatagium was supported by a single, enormously elongated finger, generally regarded as the fourth digit of the hand. The first three digits, by contrast, were short and clawed. The propatagium was supported by the pteroid bone: typically a long, slender element, unique to pterosaurs, that articulated at the wrist (figure 1a).
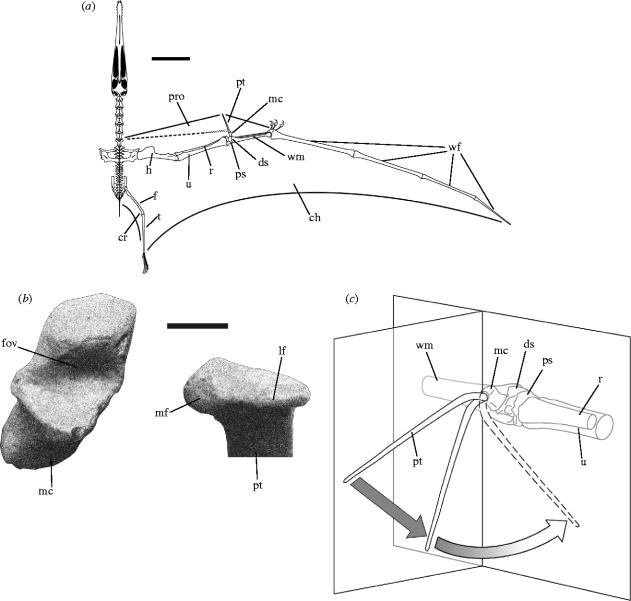
Figure 1.
Skeletal reconstruction of A. santanae. (a) Reconstructed skeleton of the right wing and membrane outlines in dorsal view, adapted from Wellnhofer (1991b), showing the pteroid in the antero-ventral orientation, supporting a broad propatagium (solid line) and in the medial orientation, supporting a narrow propatagium (broken line): scale bar=200 mm. (b) Right medial carpal in distal view and right pteroid in proximal view, showing the articular surfaces of the carpal–pteroid joint: scale bar=20 mm. (c) Right wrist in antero-medial view, showing articular motion of the pteroid. Two planes have been superimposed, intersecting at the carpal–pteroid joint—one parallel to the wing spar, one normal to it. During the initial phase of flexion the pteroid (solid line) occupies the normal plane, and angulation thus takes the form of pure depression. During the second phase the articular head of the pteroid rotates laterally with respect to the medial carpal, and angulation therefore gradually shifts from depression to adduction. The pteroid swings out of the normal plane until at the limit of flexion (broken line) it comes to occupy the parallel plane. Abbreviations: ch, cheiropatagium; cr, cruropatagium; ds, distal syncarpal; f, femur; fov, fovea of the medial carpal; h, humerus; lf, lateral facet of the pteroid; mc, medial carpal; mf, medial facet of the pteroid; pro, propatagium; ps, proximal syncarpal; pt, pteroid; r, radius; t, tibiotarsus; u, ulna; wf, wing-finger; wm, wing-metacarpal.
The function of the pteroid is one of the most contentious aspects of pterosaur palaeobiology. It is widely believed that the bone pointed towards the body, forming the distal part of the leading edge of the propatagium, because it is nearly always oriented in this way in flattened, articulated fossil skeletons (Bramwell & Whitfield 1974; Wellnhofer 1985, 1991a). This idea was challenged by Frey & Riess (1981), who argued both from an aerodynamic standpoint and by analogy with birds and bats that, had the pteroid pointed towards the body, the propatagium would have been too small and its range of movement too limited to have functioned effectively in flight. Based on their investigation of three-dimensionally preserved wrist bones from the Cambridge Greensand (Lower Cretaceous), they claimed instead that the pteroid was directed forwards and downwards (antero-ventrally) in flight, and had a substantial range of movement in a vertical plane. The propatagium envisaged by Frey & Riess was therefore much broader than had previously been believed (figure 1a), and its ventral deflection would have deeply cambered the proximal region of the wing, enabling the propatagium to act as a leading-edge flap.
This new reconstruction was criticized for several reasons. It was argued that the pteroid would have been too fragile to project ahead of the wing spar or manipulate the propatagium in flight (Padian 1984; Wellnhofer 1985). Also, as more three-dimensional fossils were discovered in the 1980s it became clear that, owing to the fragmentary nature of the Cambridge Greensand material, Frey & Riess had made a number errors in their reconstruction of the wrist, and doubt was thus cast upon their conclusions (Padian 1984; Wellnhofer 1985). The new reconstruction was, therefore, largely rejected. However, further analyses of exceptionally well preserved three-dimensional specimens of large pterodactyloid pterosaurs from the Santana Formation (Lower Cretaceous) of Brazil indicated that the pteroid could in fact have pointed both antero-ventrally and medially (Pennycuick 1988; Wellnhofer 1991b; Unwin et al. 1996). Pennycuick (1988) even suggested that the pteroid could have snapped between these orientations for slow and fast flight, respectively.
At present, it is not possible to resolve the debate about pteroid function using fossil evidence alone. We attempted to solve the problem in a different way, by comparing the aerodynamic performance of the medial and forward-pointing orientations of the pteroid using wind tunnel tests of scale models. The results of our experiments provide compelling evidence that the pteroid was directed antero-ventrally in flight, and suggest that the pteroid/propatagium complex may have played a crucial role in the evolution of giant size in pterosaurs.
2. Fossil material
We reconstructed the pterosaur wrist on the basis of two three-dimensionally preserved skeletons of ornithocheirid pterosaurs from the Santana Formation of Brazil: Anhanguera santanae (AMNH 22555), described by Wellnhofer (1991b) and Coloborhynchus robustus (SMNK 1133PAL). Specimen AMNH 22555 was a sub-adult at the time of death, whereas SMNK 1133PAL was an adult, as indicated by the state of fusion of the shoulder girdle, carpus, etc. (Bennett 1993). The wrist bones of both the left and right wings of AMNH 22555 are preserved, together with proximal ends of both pteroids. Only the right wrist is preserved in its entirety in SMNK 1133PAL, but the right pteroid is nearly complete, missing only the distal tip and a small portion of the articular condyle. All elements have been freed from the matrix and can be directly articulated. Further morphological information was obtained from the remains of other Santana Formation ornithocheirids, including ?Anhanguera (IMCF 1053, SMNK 1136PAL), ?Brasileodactylus (AMNH 24444), Coloborhynchus (NSM-PV 19892; Kellner & Tomida 2000), Santanadactylus (AMNH 22552; Wellnhofer 1991b), ?Santanadactylus (SMNK 1250PAL) and the crushed but near-complete articulated skeleton of the ornithocheirid Arthurdactylus (SMNK 1132PAL) from the Crato Formation (Lower Cretaceous) of Brazil (Frey & Martill 1994). Institutional abbreviations: AMNH, American Museum of Natural History, NY, USA; IMCF, Iwaki Coal and Fossil Museum, Iwaki, Japan; NSM, National Science Museum, Tokyo, Japan; SMNK, Staatliches Museum für Naturkunde, Karlsruhe, Germany.
3. Orientation and range of movement of the pteroid
The pterosaur carpus consists of two proximal and four distal carpals, excluding the pteroid, which may itself be a modified distal carpal (Unwin et al. 1996). The proximal carpals are fused into a proximal syncarpal in osteologically mature specimens, while three of the distal carpals fuse to form a distal syncarpal (Bennett 1993). The remaining distal carpal, referred to here as the medial carpal (Padian 1984), but which has also been termed the distal lateral (Wellnhofer 1985), or pre-axial carpal (Bennett 2001), articulates on a vertically elongate biconvex facet on the anterior surface of the distal syncarpal. The medial carpal bears a deep concave fovea that opens anteriorly, ventrally and somewhat medially, within which the pteroid articulates (figure 1b). This interpretation is not universally accepted: Bennett (2001) noted that a small oval sesamoid bone is sometimes found preserved within the fovea of the medial carpal, and argued that the pteroid did not articulate here, but on a shallow articular facet on the ventral side of the medial carpal near its base. We found no such articular facet in any of the medial carpals we examined, and therefore reject this idea. We propose instead that the sesamoid in question was originally embedded in the tendon of a pteroid extensor or flexor muscle where it passed over the medial carpal, and that it was pulled into the fovea after death in some specimens as a result of disarticulation of the carpal–pteroid joint.
The fovea of the medial carpal is asymmetrical, having the shape of a portion of the inner surface of a cone, the apex pointing laterally and the base medially. The convex articular surface of the pteroid is elongated medially and is similarly asymmetrical, with a broad, subtriangular medial facet and a narrow, roller-like lateral facet (figure 1b). The head of the pteroid is offset ventrally from the shaft by 30° and medially by 10°. At maximum extension the shaft of the pteroid points forwards, about 10° beneath the horizontal plane (figure 1c). In this position a shallow semicircular facet on the dorsal surface of the pteroid, just distal to the articular head (Unwin et al. 1996), fits tightly against the upper part of the articular surface of the medial carpal, which acts as a bony stop, preventing further extension. As the joint is flexed, the large medial facet of the head of the pteroid slides dorsally around the inside medial edge of the fovea, while the narrow, lateral facet rolls in place. Hence, during flexion, the head of the pteroid undergoes a lateral rotation of 20° (the sense being clockwise for the right pteroid viewed proximally). This rotation is conjunct, i.e. it is an indissociable effect of articular movement. Flexion of the pteroid is therefore arcuate, with an initial depression giving way to adduction as the flexural limit is approached (figure 1c). At this limit, the shaft of the pteroid points ventro-medially, 45° beneath the horizontal plane. Further flexion is prevented by the ventral lip of the fovea of the medial carpal, which acts as another bony stop. The pteroid can also be articulated such that the shaft is directed medially, parallel to the transverse axis, but in this case only a fraction of the area of the carpal–pteroid joint surfaces are utilized, primarily the medial facet of the pteroid and the ventral region of the fovea of the medial carpal. The poor articular fit indicates that the likelihood of such an orientation is low. Regardless, it is not possible for the pteroid to move from a forward-pointing orientation to a purely medial orientation without first disarticulating the joint.
4. Experimental methods
(a) Wing reconstruction
In order to place the wrist in context, we reconstructed the wing of A. santanae (AMNH 22555) in three dimensions. The axial skeleton of this specimen is complete, but only fragments of the limbs are preserved. We therefore measured the specimens listed above to determine the relative dimensions of the wing bones in the Ornithocheiridae, and estimated bone lengths of A. santanae accordingly. We then directly articulated the major joints, each of which is represented in at least one of the specimens examined, to determine individual joint angles and from these the three-dimensional spatial configuration of the wing skeleton.
The wing membranes were added, assuming that the leading edge of the propatagium ran from the neck to the tip of the pteroid, thence to the knuckle, and that the trailing edge of the cheiropatagium ran to the distal end of the lower leg (figure 2a). This condition of the cheiropatagium is evident in soft tissue fossils of several unrelated pterosaur species (Unwin & Bakhurina 1994; Lu 2002; Wang et al. 2002; Frey et al. 2003), and it is therefore most parsimonious to conclude that it is universal for pterosaurs, given the absence of firm evidence to the contrary (Unwin 1999).
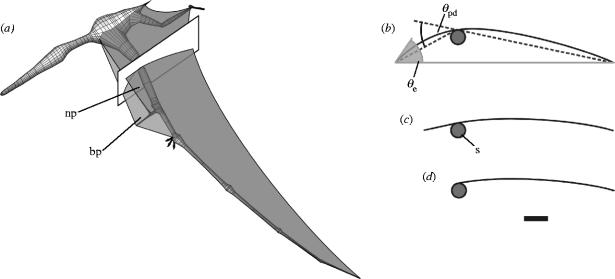
Figure 2.
Cross-sections of the wing of A. santanae. (a) Three-dimensional reconstruction of the fleshed-out wing skeleton, cheiropatagium, cruropatagium, and the narrow and broad reconstructions of the propatagium, indicating the plane of the cross-section used for the wind tunnel models. (b–d) Wing sections, with (b) broad, (c) narrow and (d) no propatagium. All three sections are shown at an angle of attack of 0°. Scale bar=50 mm. Abbreviations and symbols: bp, broad propatagium; np, narrow propatagium; s, spar; θe, entry angle; θpd, propatagium deflection angle.
(b) Wind tunnel models
We determined the aerodynamic consequences of the forward-pointing and medial orientations of the pteroid by carrying out wind tunnel tests of profile models representing a cross-section of the reconstructed wing. We took the cross-section in question halfway between the shoulder and wrist. At this station we determined the relative positions of the trailing edge of the cheiropatagium, the wing spar, and the leading edge of the propatagium.
Three models were constructed: one with a broad propatagium (figure 2b), corresponding to the forward-pointing pteroid orientation, one with a narrow propatagium (figure 2c), corresponding to the medial pteroid orientation, and one in which the propatagium was omitted (figure 2d). Each consisted of a membrane of ripstop nylon—an inextensible close-weave fabric—and a supporting framework. Model span was equal to the width of the tunnel test section, and the cross-section of each model was identical along the span. The dimensions of the model cross-sections were obtained by scaling down the dimensions of the wing cross-section by a factor of two (table 1).
Table 1.
Model dimensions.
| model | chord (mm) | span (mm) | spar diameter (mm) | spar-leading edge (mm) | spar-trailing edge (mm) |
|---|---|---|---|---|---|
| no forewing | 173 | 706 | 16 | — | 166 |
| narrow forewing | 198 | 706 | 16 | 32 | 166 |
| broad forewing | 218–239 | 706 | 16 | 73 | 166 |
The model framework consisted of an aluminium cylinder (representing the wing spar), leading and trailing edge supports, pairs of endplates at either end of the spar to hold the supports, and a posteriorly directed rod (sting) to suspend the model in the wind tunnel. The supports were each made of three 3 mm diameter steel rods soldered together along their length. They were rigid in order to satisfy the requirement for an identical profile along the span (Greenhalgh et al. 1984; Newman & Low 1984; Sugimoto & Sato 1991). The endplates for the leading edge supports could be rotated around the spar to alter the ventral deflection angle of the propatagium, θpd (see figure 2b). The broad propatagium model was tested with deflection angles between 10° and 60°, in ten-degree increments, to determine the effects of pteroid flexion. The deflection angle of the narrow propatagium model was set at 15°, as estimated from the reconstruction.
Given that the material properties of the pterosaur wing membrane are unknown, we simulated the effects of differing membrane elasticity by varying the slackness of the nylon fabric. This is a valid approach, as increased elasticity of a membrane profile merely increases camber for a given aerodynamic loading, and is equivalent to increasing the slackness of an inextensible membrane (Jackson 1983). Slackness was quantified using the excess length ratio parameter ϵ, defined as the difference between membrane length and chord, divided by the chord. Models were tested with excess length ratios of 0.02, 0.04 and 0.06, a typical range for sails (Greenhalgh et al. 1984; Newman & Low 1984; Sugimoto & Sato 1991).
(c) Wind tunnel tests
We used an open circuit wind tunnel with a closed test section 0.71 m wide by 0.51 m high, located in the Department of Engineering, University of Cambridge. It is equipped with a three-component force balance that measures lift, drag and pitching moment (the latter component was not of interest here). The measured drag comprised only profile drag, caused by air resistance. Induced drag, caused by the flow of air around the wingtips, was eliminated because the models spanned the width of the test section, so that the tunnel walls prevented such flow. Models were run at a Reynolds number of 1.2×105, estimated by applying a method developed for Pteranodon ingens (Bramwell & Whitfield 1974) to the reconstruction of A. santanae (Wilkinson 2002). Lift and drag measurements were taken at angles of attack between −2° and 20° in two-degree increments. However, owing to model instabilities, it was not possible to take measurements at angles of attack of −2° and 20° for the narrow propatagium model, or angles of attack of −2°, 0° and 20° for the model without a propatagium. The measured drag of the models included the drag of the supporting endplates, sting and suspension wires. This support drag was quantified by running the framework in the wind tunnel without any membranes, and was then deducted from the measured values to obtain the true profile drag. Lift and profile drag were converted into their respective dimensionless coefficients thus
| 4.1 |
| 4.2 |
where CL and CD,pro are the lift and profile drag coefficients, L and Dpro are the lift and profile drag forces in N, ρ is air density in kg m−3, V is relative air velocity in m s−1 and S is wing area (model span×chord) in m2.
5. Experimental results
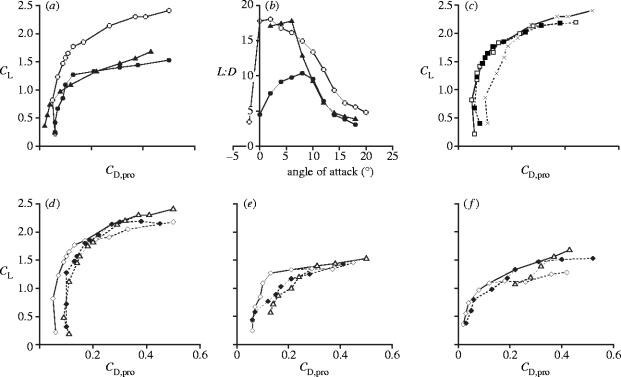
In terms of lift production (figure 3a) the broad forewing model was clearly superior to the others, developing exceptionally high lift coefficients (CL) at high angles of attack: most conventional aerofoils and sail profiles have maximum CL values of about 1.5 (Greenhalgh et al. 1984; Newman & Low 1984; Selig et al. 1989; Sugimoto & Sato 1991), as opposed to the CL,max of 2.4 recorded here. A comparison with birds and bats is not possible, because profile data have not been directly obtained for these animals. It should be stressed that the increased lift coefficient of the broad propatagium model was a result of its different cross-sectional shape, not its increased area, as the lift coefficient is standardized with respect to wing area (equation (4.1)). The lift : drag (L : D) ratios (figure 3b) of the broad propatagium model were also superior to those of the narrow propatagium model, particularly at low angles of attack, where the maximum L : D reaches 18.1. The narrow propatagium model performed surprisingly badly: even when compared with the model without a propatagium there was no improvement in terms of either L : D or CL.
Figure 3.
Wind tunnel results for the pterosaur wing sections. (a) Polar diagram, in which the lift and profile drag forces measured for each model have been converted into their respective dimensionless coefficients, CL and CD,pro (equations (4.1) and (4.2)), and plotted against each other. Each point represents a CL and CD,pro value measured at a single angle of attack, and angle of attack increases from −2° at the bottom left to 20° at the top right. Open circles, broad forewing model; closed circles, narrow forewing model; triangles, model without a forewing. (b) L : D ratios. Symbols as in (a). (c) Polar diagram showing the effects of variation in θpd on the performance of the broad propatagium model. Open squares, θpd=30°; closed squares, θpd=40°; crosses, θpd=50°. The solid line indicates the best performance over the entire angle of attack range, and it is this composite polar for the broad propatagium model that has been plotted in (a). (d–f) Polar diagram showing the effects of variation in ϵ on the performance of (d) the broad propatagium model, (e) the narrow propatagium model and (f) the model without a propatagium. Open diamonds, ϵ=0.02; closed diamonds, ϵ=0.04; open triangles, ϵ=0.06. The solid lines again indicate the best performance, and are plotted in (a).
To our knowledge, this is the first investigation of the aerodynamic consequences of the addition of a leading edge flap to a membrane aerofoil. The effects are somewhat similar to those seen when such a device is added to a conventional aerofoil (Fullmer 1947; Applin et al. 1995). The remarkably high lift characteristics of the broad propatagium model were the result of its deep camber, or more specifically, its large entry angle, θe (figure 2b), this being the angle between a tangent at the leading edge and the chord line (an imaginary straight line running from the leading edge to the trailing edge). The large entry angle meant that the propatagium became aligned with the airflow at high angles of attack so that, contrary to expectation, the wing section did not stall. This inference was verified by using smoke trails to visualize the streamlines: even at the highest angles of attack the flow was still attached to the propatagium. Increasing the deflection angle of the propatagium further increased the angle of attack at which stalling took place, and further increased the lift (figure 3c), though only up to a point: deflection angles beyond 50° caused excessive flow separation from the posterior part of the upper surface of the wing and gave no additional benefit. At low angles of attack a sharply deflected propatagium obstructed the airflow, so that smaller deflection angles improved performance here (figure 3c), although once again the improvement was limited: if the deflection angle was less than 30° the reduced flow obstruction by the propatagium was outweighed by flow separation at the wing spar. A propatagium deflection of 30°–40° gave the best performance over the mid-range angles of attack—between 2° and 16° (figure 3c).
Entry angle was also increased a little when the membrane was slackened (i.e. when ϵ was increased), which slightly improved the performance of all three models at higher angles of attack (figure 3d–f). The effect was, however, not as pronounced as that resulting from the more substantial increase in entry angle brought about by the addition of the leading edge flap. The slackness of a simple membrane aerofoil would have to be greatly increased for the entry angle to approach that of an aerofoil with a sharply deflected propatagium. The camber of such a profile would be so large that any benefit associated with the large entry angle would be outweighed by the substantial flow separation that would inevitably result (Jackson & Fiddes 1995).
6. Discussion
Our findings strongly support the idea that the pteroid was directed antero-ventrally during flight, given the greatly increased maximum CL and L : D associated with the broad, ventrally deflected propatagium. Both parameters are biologically important because they determine, respectively, gliding speed and glide angle: a high CL,max gives a low minimum gliding speed, and a high L : D gives a shallow glide angle, and a greater gliding range. The pteroid would probably have been fully flexed only to furl the propatagium when on the ground, a likely necessity given the membrane's large size. It is this furled configuration that can be seen in articulated fossils. The pteroid could not have snapped between medial and forward-pointing orientations in flight as suggested by Pennycuick (1988) because, quite apart from the poor performance of the medial orientation, this movement was not permitted by the morphology of the carpal–pteroid joint.
It has been argued that a forward-pointing pteroid would have been too fragile to withstand the aerodynamic loads imposed on it during flight and when manipulating the propatagium, or the compressive stress caused by tension in the propatagium and its leading edge tendon, if such a tendon existed (Padian 1984; Wellnhofer 1985). This is very unlikely: the stresses borne by the pteroid would have been insignificant compared with those borne by the equally long and slender fourth phalanx of the wing-finger, particularly during a wingbeat. One also need only consider the wing digits of a rapidly flapping bat wing to appreciate how resilient such apparently fragile structures can be.
In our reconstruction of the wing of A. santanae we have included a propatagial membrane distal to the wrist, reasoning that the propatagium could not have terminated at the pteroid: if it had, there would have been an abrupt change in the wing's cross-section at this point, which seems unlikely on aerodynamic grounds. Frey & Riess (1981) similarly reconstructed such a membrane (although their propatagium extended even further out along the wing and attached to the three short, clawed digits). Some workers rejected this idea, contending that there was no evidence for this distal propatagium (Wellnhofer 1987; Padian & Rayner 1993). This claim is incorrect. The superbly preserved ‘Zittel wing’, an isolated wing, assigned to Rhamphorhynchus, from the Solnhofen Limestone (Upper Jurassic; von Zittel 1882), and an unidentified large pterodactyloid from the Crato Formation (Lower Cretaceous; Frey & Tischlinger 2000) both have traces of membrane distal to the wrist. The apparent lack of such a membrane in many fossils can be readily explained. In those articulated specimens (mainly from the Solnhofen Limestone) in which the outline of the propatagium can be clearly discerned, the pteroid has been fully flexed, which would have caused the distal propatagium to become tightly folded against the metacarpus. As such, it could easily have been obscured by the skeleton or the soft tissues of the hand, or removed during preparation of the metacarpus. We concur with Wellnhofer (1985) that there is no good reason to suppose that the propatagium attached to the three short, clawed fingers. In many specimens with good soft part preservation no remnants of a membrane in the vicinity of the fingers have been found.
When Frey & Riess (1981) first presented their ideas, Padian (1984) and Wellnhofer (1985) commented that the traditional reconstruction of the pteroid had not been shown to be aerodynamically deficient. Our wind tunnel tests show clearly that a pterosaur wing profile with a narrow propatagium is aerodynamically inferior to one with a broad leading-edge flap. We can be confident that the same would have been true for complete pterosaur wings: as stated above, the propatagium probably extended from the wing root to the knuckle, a region of the wing that, in ornithocheirids, represented over half its projected wing area (Wilkinson 2002).
The high lift capability of pterosaur wings revealed by our wind tunnel tests offers a solution to the problem of how the largest members of the group managed to get airborne. Although many must have used gravity-assisted take-off from cliffs, the localities of some fossil finds (Lawson 1975) and trackway evidence (Hwang et al. 2002) suggest that some large forms occasionally took off from level ground. It is difficult to envisage how these giants could have developed sufficiently rapid airflow over their wings to lift their bulk, partly because, in all likelihood, they had only a limited flapping ability (Bramwell & Whitfield 1974; Alexander 1998), but also because a fast running take-off would have been hindered by the attachment of the wing membranes to the legs. However, the remarkably high CL,max of the wings, coupled with large pterosaurs' low wing loading, i.e. weight divided by wing area (Bramwell & Whitfield 1974; Alexander 1998), may have lowered the minimum gliding speed to the point at which even the largest forms needed only to spread their wings while facing into a moderate breeze in order to take off. In this regard, it may be significant that the Azhdarchidae, the family containing these giants, are characterized by the possession of relatively short wing-fingers (Unwin 2003a). Therefore, the spanwise extent of the propatagium was unusually great in these forms, increasing their high-lift capability.
The ventrally deflected propatagium would also have circumvented another potential problem that was caused by the attachment of the legs to the wing membranes. If, during landing, the legs were brought into contact with the ground while the wings were still outstretched, as seems likely, then the angle of attack of the proximal wing would have been greatly increased and the wing liable to stall. The deflected forewing would have eliminated this problem by maintaining airflow over the wing even at very high angles of attack. These functions of the adjustable propatagium—the augmentation of lift and prevention of stall during take-off and landing—are similar to those of the flaps on an aircraft wing or the alula of birds, though the way in which high lift is obtained is very different in the latter case (Graham 1932).
The pteroid and its associated propatagium clearly played a critical role in the flight of the giant pterosaurs. It is important to note, however, that the pteroid occurred in all pterosaurs, many of which were of a more modest size. It is now widely accepted that these smaller forms were fully capable of flapping flight (Padian 1983; Hazlehurst & Rayner 1992), and probably would not have had any difficulty taking off or landing, regardless of the orientation of the pteroid. However, lift-enhancement was not the sole function of the leading edge flap: it would also have increased drag during landing, thus acting as an airbrake, and would have served as a control surface during normal flight. For example, flexing one pteroid while extending the other would have increased lift on one wing, thereby initiating a roll. These features add to an increasing amount of evidence (Frey et al. 2003; Unwin 2003b; Witmer et al. 2003) demonstrating that, although long extinct, the pterosaurs were exceptionally competent and capable fliers that possessed a highly sophisticated, and above all unique flight apparatus.
Acknowledgments
We thank M. Manabe, S. Nabana, Y. Takakuwa, E. Frey, M. A. Norell and E. S. Gaffney for access to material; H. Babinsky, D. G. Nunn, J. R. Clarke, D. S. Martin and B. Stock for assistance with the wind tunnel tests; S. J. Ellis and R. E. D. Holder for helping to construct the models, and A. E. Friday and N. Bakhurina for commenting on earlier versions of the manuscript. The work was supported by a BBSRC research studentship. MTW is supported by a Junior Research Fellowship from Clare College, University of Cambridge.
References
- Alexander R.M. All-time giants: the largest animals and their problems. Palaeontology. 1998;41:1231–1245. [Google Scholar]
- Applin, Z. T., Gentry, G. L. & Takallu, M. A. 1995 Wing pressure distributions from subsonic tests of a high-wing transport model. NASA-TM4583.
- Bennett S.C. The ontogeny of Pteranodon and other pterosaurs. Paleobiology. 1993;19:92–106. [Google Scholar]
- Bennett S.C. The osteology and functional morphology of the Late Cretaceous pterosaur Pteranodon. Palaeontographica A. 2001;260:1–153. [Google Scholar]
- Bramwell C.D, Whitfield G.R. Biomechanics of Pteranodon. Phil. Trans. R. Soc. B. 1974;267:503–581. [Google Scholar]
- Frey E, Martill D.M. A new pterosaur from the Crato Formation (Lower Cretaceous, Aptian) of Brazil. N. Jb. Geol. Paläont. Abh. 1994;194:379–412. [Google Scholar]
- Frey E, Riess J. A new reconstruction of the pterosaur wing. N. Jb. Geol. Paläont. Abh. 1981;161:1–27. [Google Scholar]
- Frey E, Tischlinger H. Weichteilanatomie der Flugsaurierfüße und Bau der Scheitelkämme: Neue Pterosaurierfunde aus den Solnhofener Schichten (Bayern) und der Crato-Formation (Brasilien) Archaeopteryx. 2000;18:1–16. [Google Scholar]
- Frey E, Tischlinger H, Buchy M.-C, Martill D.M. New specimens of Pterosauria (Reptilia) with soft parts with implications for pterosaurian anatomy and locomotion. In: Buffetaut E, Mazin J.-M, editors. Evolution and palaeobiology of pterosaurs. Special Publications 217. Geological Society of London; London: 2003. pp. 233–266. [Google Scholar]
- Fullmer, F. F. 1947 Two-dimensional wind-tunnel investigation of the NACA 641-012 airfoil equipped with two types of leading-edge flap. NACA-TN1277.
- Graham R.R. Safety devices in wings of birds. Aeronaut. J. 1932;36:24–58. [Google Scholar]
- Greenhalgh S, Curtiss H.C, Smith B. Aerodynamic properties of a two-dimensional inextensible flexible airfoil. AIAA J. 1984;22:865–870. [Google Scholar]
- Hazlehurst G.A, Rayner J.M.V. Flight characteristics of Triassic and Jurassic Pterosauria—an appraisal based on wing shape. Paleobiology. 1992;18:447–463. [Google Scholar]
- Hwang K.-G, Huh M, Lockley M.G, Unwin D.M, Wright J.L. New pterosaur tracks (Pteraichnidae) from the Late Cretaceous Uhangri Formation, southwestern Korea. Geol. Mag. 2002;139:421–435. 10.1017/S0016756802006647 [Google Scholar]
- Jackson P.S. A simple model for elastic two-dimensional sails. AIAA J. 1983;21:153–155. [Google Scholar]
- Jackson P.S, Fiddes S.P. Two-dimensional viscous flow past flexible sail sections close to ideal incidence. Aeronaut. J. 1995;99:217–225. [Google Scholar]
- Kellner A.W.A, Tomida Y. Description of a new species of Anhangueridae (Pterodactyloidea) with comments on the pterosaur fauna from the Santana Formation (Aptian–Albian), Northeastern Brazil. Nat. Sci. Mus. Monogr. 2000:17. [Google Scholar]
- Lawson D.A. Pterosaur from the latest Cretaceous of west Texas: discovery of the largest flying creature. Science. 1975;187:947–948. doi: 10.1126/science.187.4180.947. [DOI] [PubMed] [Google Scholar]
- Lu J.-C. Soft tissue in an early Cretaceous pterosaur from Liaoning province, China. Mem. Fukui Pref. Dinosaur Mus. 2002;1:19–28. [Google Scholar]
- Newman B.G, Low H.T. Two-dimensional impervious sails: experimental results compared with theory. J. Fluid Mech. 1984;144:445–462. [Google Scholar]
- Padian K. A functional analysis of flying and walking in pterosaurs. Paleobiology. 1983;9:218–239. [Google Scholar]
- Padian K. A large pterodactyloid pterosaur from the two medicine formation (Campanian) of Montana. J. Vert. Paleontol. 1984;4:516–524. [Google Scholar]
- Padian K, Rayner J.M.V. The wings of pterosaurs. Am. J. Sci. 1993;293:91–166. [Google Scholar]
- Pennycuick C.J. On the reconstruction of pterosaurs and their manner of flight, with notes on vortex wakes. Biol. Rev. 1988;63:299–331. [Google Scholar]
- Selig M.S, Donovan J.F, Fraser D.B. SoarTech Publications; Virginia Beach: 1989. Airfoils at low speeds. [Google Scholar]
- Sugimoto T, Sato J. Aerodynamic characteristics of two-dimensional membrane airfoils. Trans. Jpn. Soc. Aeronaut. Space Sci. 1991;34:88–100. [Google Scholar]
- Unwin D.M. Pterosaurs: back to the traditional model? Trends Ecol. Evol. 1999;14:263–268. doi: 10.1016/s0169-5347(99)01605-5. 10.1016/S0169-5347(99)01605-5 [DOI] [PubMed] [Google Scholar]
- Unwin D.M. On the phylogeny and evolutionary history of pterosaurs. In: Buffetaut E, Mazin J.-M, editors. Evolution and palaeobiology of pterosaurs. Special Publications 217. Geological Society; London: 2003a. pp. 139–190. [Google Scholar]
- Unwin D.M. Smart-winged pterosaurs. Nature. 2003b;425:910–911. doi: 10.1038/425910b. 10.1038/425910b [DOI] [PubMed] [Google Scholar]
- Unwin D.M, Bakhurina N.N. Sordes pilosus and the nature of the pterosaur flight apparatus. Nature. 1994;371:62–64. 10.1038/371062a0 [Google Scholar]
- Unwin D.M, Frey E, Martill D.M, Clarke J.B, Riess J. On the nature of the pteroid in pterosaurs. Proc. R. Soc. B. 1996;263:45–52. [Google Scholar]
- von Zittel K.A. Ueber Flugsaurier aus dem lithographischen Schiefer Bayerns. Palaeontographica. 1882;29:47–80. [Google Scholar]
- Wang X.-L, Zhou Z.-H, Zhang F.-C, Xu X. A nearly completely articulated rhamphorhynchoid pterosaur with exceptionally well-preserved wing membranes and ‘hairs’ from Inner Mongolia, northeast China. Chin. Sci. Bull. 2002;47:226–230. 10.1360/02tb9054 [Google Scholar]
- Wellnhofer P. Neue pterosaurier aus der Santana-Formation (Apt) der Chapada do Araripe, Brasilien. Palaeontographica A. 1985;187:105–182. [Google Scholar]
- Wellnhofer P. Die Flughaut von Pterodactylus (Reptilia, Pterosauria) am Beispiel des Wiener Exemplares von Pterodactylus kochi (Wagner) Ann. Naturhist. Mus. Wien. 1987;88A:149–162. [Google Scholar]
- Wellnhofer P. Salamander Books Ltd; London: 1991a. The illustrated encyclopedia of pterosaurs. pp. 54, 147. [Google Scholar]
- Wellnhofer P. Weitere Pterosaurierfunde aus der Santana-Formation (Apt) der Chapada do Araripe, Brasilien. Palaeontographica A. 1991b;215:43–101. [Google Scholar]
- Wilkinson, M. T. 2002 Flight of the ornithocheirid pterosaurs. Ph.D. thesis, University of Cambridge.
- Witmer L.M, Chatterjee S, Franzosa J, Rowe T. Neuroanatomy of flying reptiles and implications for flight, posture and behaviour. Nature. 2003;425:950–953. doi: 10.1038/nature02048. 10.1038/nature02048 [DOI] [PubMed] [Google Scholar]