Abstract
Sperm competition models predict that males typically mating in disfavoured roles should be selected to compensate for their disadvantage by investing more into sperm. We studied the effect of rapid changes in social status on ejaculate investments during experimental trials with an externally fertilizing teleost—the Arctic charr (Salvelinus alpinus). We document that males becoming dominant produce less sperm with lower velocity, but have higher sex steroid concentrations than subordinate males. These differences in sperm characteristics seem mainly to result from a decreased investment in sperm among fish that become dominant compared to pre-trial levels. Moreover, these adjustments of sperm production and sperm velocity seem not to be traded against sperm longevity. Our results support theoretical models of sperm competition, as males forced to mate in disfavoured roles seem to invest more into ejaculate quality than males in favoured roles. Additionally, we are the first to report that males, in a species with status-dependent shifts in reproductive tactics, have evolved rapid tactic specific adjustments of sperm production and sperm velocity corresponding to what could be predicted from their reproductive roles.
Keywords: alternative mating tactics, sperm competition, sperm motility, sperm velocity, evolution, sex steroids
1. Introduction
In species with external fertilization, males' reproductive success is less influenced by female manipulation, such as selective storage, removal or displacement of sperm, compared to males in species with internal fertilization (Eberhard 1996; Birkhead & Møller 1998). Thus, in external fertilizers, sperm from males spawning in favoured roles (i.e. in synchrony with the female and close to her eggs) are believed to have precedence over sperm from males spawning in disfavoured roles (i.e. out of synchrony with females or further away from the eggs) in the same spawning event (Birkhead & Møller 1998). In such species, males occupying disfavoured mating roles seem, however, to compensate by having larger gonads for their body size compared to males mating in favoured roles (Taborsky 1998, see however Liljedal & Folstad 2003). Males in disfavoured roles may also increase sperm numbers released in the ejaculate compared to males in favoured roles (Parker 1990; Parker 1993; Gage et al. 1995). In a recent review, DeWoody & Avise (2001) found that nest-holding males seem to have a higher probability of fathering offspring than sneaking males. This indicates that individuals in species with status-dependent shifts in reproductive tactics, should shift their reproductive tactic from sneaking to social dominance, if costs of achieving dominance are not offset by costs to future reproductive value (Stearns & Hoekstra 2000). Species with such status-dependent shifts in reproductive tactics are common, and outnumber those with fixed reproductive tactics (Gross 1996).
Theoretical models suggest that sperm competition may favour males having high sperm velocity (Ball & Parker 1996) and empirical studies show that sperm velocity predicts fertilization success (Birkhead et al. 1999; Froman et al. 1999; Levitan 2000; Al-Qarawi et al. 2002; Gage et al. 2004). In external fertilizing species were fertilization occurs just a few seconds after the gametes are released (Iwamatsu et al. 1991; Hoysak & Liley 2001; Liley et al. 2002), increased sperm velocity, rather than longevity, should be an advantage in sperm competition. This is observed in bluegills (Lepomis macrochirus) where males in disfavoured mating roles have faster initial sperm swimming speed, but shorter sperm longevity than parental males holding nests sites (Neff et al. 2003; Burness et al. 2004, but see Burness et al. 2005). Yet, empirical studies on fish report ambiguous results regarding differences in sperm velocity and sperm longevity between males in different spawning roles (Defraipont et al. 1993; Gage et al. 1995; Uglem et al. 2001; Vladic & Jarvi 2001; Neff et al. 2003; Burness et al. 2004; Kortet et al. 2004), questioning the generality of a trade-off between sperm velocity and longevity.
Androgens are involved in the development and maintenance of male reproductive traits, most notable spermatogenesis, but also in reproductive behaviour and ornamental development (Brantley et al. 1993; Borg 1994; Schulz & Miura 2002). Among fish, males mating in favoured roles, generally, have higher levels of 11-ketotestoserone (11-KT) than males mating in disfavoured roles (Brantley et al. 1993; Pankhurst 1995). Yet, no obvious difference between the two mating tactics has been found for testosterone (T) concentrations (Brantley et al. 1993). 11-KT and T are produced by the testis in response to stimulation by gonadotropins, and T may indirectly stimulate the production of 17 alpha, 20 beta-dihydroxy-4-pregnen-3-one (17,20β-P), a sperm maturation and motility-inducing steroid (Borg 1994; Thomas et al. 1997; Yueh & Chang 1997; Antonopoulou et al. 1999). 17,20β-P is suggested to mediate sperm motility through an increase in the seminal plasma pH, which in turn increase the sperm content of cAMP (Miura et al. 1991, 1995). Moreover, as androgens suppress immune responses (Grossman 1984; Folstad & Karter 1992; Slater et al. 1995, reviewed in Klein 2000) they may aid the production of high quality ejaculates, as sperm are non-self and exposed to immunological targeting in the male reproductive tract (Folstad & Skarstein 1997; Hillgarth et al. 1997; Skau & Folstad 2003, 2004; Turek et al. 1996). High levels of immunosuppressive androgens may consequently prevent or hinder the negative effects from immune responses targeted towards sperm cells, such as binding of specific antibodies which may reduce sperm velocity (Grossman 1984; Slater et al. 1995; Skau & Folstad 2003, 2004). In sum, high levels of plasma androgens should, according to theory, correlate with increased sperm quality.
The Arctic charr (Salvelinus alpinus), is suitable for exploring sperm competition theory. It has external fertilization and a lek-like spawning behaviour where males offer no shelter where spawning can occur isolated from sneaking males. Further, both males and females mate with several mates during the spawning period (Fabricius & Gustafson 1954). Moreover, males have reproductive roles, i.e. different social status, that is easily identified (Sigurjonsdottir & Gunnarsson 1989) and maintaining dominance is documented to be energetically costly for males (Cutts et al. 2001). Dominant males also have lower density of sperm cells and less numbers of sperm cells for given size than subordinate males (Liljedal & Folstad 2003). Thus, it seems as dominant males invest less in sperm quantity and sperm quality compared to subordinate males. As males may shift social status depending on the presence or absence of other interacting males (Sigurjonsdottir & Gunnarsson 1989), change in status may occur rapidly and influence ejaculate characteristics (Liljedal & Folstad 2003).
The primary aim of this study was to examine whether males that attain low social status, and therefore are more likely to experience sperm competition, increase their ejaculate investments compared to males that attain high social status (Ball & Parker 1996; Parker 1993). That is, could ejaculate characteristics be predicted from the attained social status. We examined differences in sperm velocity, frequency of motile cells, spermatocrit and ejaculate volume between size-matched pairs of wild-caught male Arctic charr both before and after they had settled their social status (i.e. either dominant or subordinate) in an experimental enclosure. Additionally, we examined the association between plasma sex steroid concentrations and ejaculate characteristics. Theory suggests that high levels of androgens should have a positive relationship with ejaculate quality (Folstad & Skarstein 1997; Hillgarth et al. 1997).
2. Material and methods
(a) Sampling and handling
During six nights, in mid September 2003, we gill netted 48 reproductively active male Arctic charr at one spawning ground in lake Fjellfrøsvatn, northern Norway (69°4′N, 19°20′E). The fish stayed in the gill nets less than 15 min and individuals with any signs of injuries were excluded from the sample. On the following day, we anaesthetized the fish using benzocain (10–12 ml benzocain solution per 10 l water) and measured fork length (nose to caudal cleft, mean 25.5, range 21.4–31.5) to the nearest millimetre and tagged each fish on the dorsal fin with a white plastic tag attached with Floy's elastic vinyl filament. Thereafter, we dried its abdomen (to avoid water contamination and activation of the sperm cells) and collected the milt by gentle bilateral abdominal pressure. The fishes were then size matched and caged in pairs, with a maximum length difference within each pair of 6 mm. The cages, made of chicken-wire (40×60×90 cm3) were placed at about 1.5 m depth, 2–3 m apart. The fish were then left undisturbed for approximately 24 h before starting observations (see § 2b). On day four, the fish were brought to the laboratory, where they were killed in a random order with a stroke to the head. Thereafter, we obtained a second samples of sperm (following the procedure described above) produced during the experimental period.
(b) Social position
Observations of fish interactions were done using water-glasses. By counting the individual number of aggressive acts (i.e. a bite or an initiation of a chase) during 5 min periods, we determined the social rank in each of the 24 pairs. Different observers observed each cage twice a day (midday and evening) for three consecutive days (30 min observation period altogether). The male performing most aggressive acts within a pair was considered the dominant one. Social dominance is easily determined and only three of the 24 subordinate individuals performed aggressive acts at all. The data from the different observers were pooled as repeatability between observers is shown to be high. See Liljedal & Folstad (2003) for a detailed evaluation of the methods applied.
(c) Ejaculate quality
To reduce overall handling time, all ejaculate measurements were conducted in the sequence the fish were handled. Milt volume was estimated in 1 ml syringes to the nearest 0.1 ml and the milt was thereafter stored at 4 °C in closed 1.5 ml Eppendorf tubes. Spermatocrit measurements and video-recordings of the sperm movements were done within 2 h after the milt was collected. Spermatocrit, which is the percentage of a given volume of milt that is occupied by cells, was measured by centrifuging about 10 μl homogeneous milt in a capillary tube for 195 s at 11 500 rpm with a Compur mini-centrifuge (Compur-electronic Gmbh, Munich, Germany). Video recordings of swimming sperm were made using a CCD black and white video camera (XC-ST50CE PAL, Sony, Tokyo, Japan) mounted on a negative phase-contrast microscope (Olympus CH30, Olympus, Tokyo, Japan) with a 10× objective. Motility was initiated by adding 4.5 μl water after placing less than 0.12 μl of sperm on a cooled (5–7 °C) standard counting chamber (Leja products BV, Nieuw-Vennep, The Netherlands). Sperm movement was recorded from activation until movement ceased (between 60 and 90 s). Each male is represented by one recording, that has evenly distributed sperm cells with a total cell-number close to 100 (mean 108±46 s.d. before caging, mean 86±36 s.d. after caging, both at 15 s post-activation). The recordings were later analysed using computer assisted sperm analysis (HTM-CEROS sperm tracker, CEROS v.12, Hamilton Thorne Research, Beverly, MA, USA), which has been shown to be an objective tool for studying sperm motility in fish (Kime et al. 1996, 2001). The image analyser was set at; frame rate 50 Hz, no of frames 25, minimum contrast 10 and minimum cell size 5 pixels. For each male we quantified sperm motility at 15, 20, 30, 40 and 50 s following activation of the sperm cells. Each motility measurement last for 0.5 s. The parameters assessed were: mean average path velocity (VAP), mean straight line velocity (VSL), mean curvilinear velocity (VCL) and percent motile cells. To remove the possible effect of drift, cells having a VAP<10 μm s−1 and a VSL<20 μm s−1 were excluded from the motility analysis.
We analysed sperm velocity both before and after the experimental period and found no significant three-way interaction between different sperm velocity measurements (VAP, VSL and VCL), time since sperm activation (15–50 s) and whether males were dominant or subordinate (repeated measurements ANOVA, before, F8,360=0.40, p=0.92 and after, F8,344=0.27, p=0.98). Thus, change in the sperm velocity following activation does not differ depending on which of the three different sperm velocity measurements used. Since in this study there were neither eggs nor ovarian fluid gradients that could have attracted or guided the sperm cells, cell trajectories were not expected to be linear and final analyses were, therefore, performed on measures of the actual point-to-point track followed by the cells (VCL).
(d) Plasma sex steroids
Blood samples were taken from the caudal sinus of freshly killed fish using preheparinised needles and blood collection tubes from the Vacutainer system. The samples were centrifuged in 1.5 ml Eppendorf tubes for 10 min at 10 000 rpm. Plasma was removed and stored in 1.5 ml Eppendorf tubes at −20 °C until analysis. Plasma concentrations of 11-KT and T were measured by means of radioimmunoassay (RIA), according to Schulz (1985). A validation of the assays for Arctic charr plasma, and cross-reactivities of the T antiserum, has previously been examined (Frantzen et al. 2004). Cross-reactivity of the 11-KT antiserum is given by Schulz (1985). Plasma concentration of 17,20β-P was measured by the same procedure as the other steroids. The suitability of the RIA protocol for measuring 17,20β-P in charr has previously been examined by Mayer et al. (1992) and cross-reactivity of the 17,20β-P antiserum is given by Scott et al. (1982). Briefly, steroids were extracted from 200 μl plasma with 4 ml diethylether under vigorously shaking for 4 min. The aqueous phase was frozen in liquid nitrogen, whereas the organic phase was transferred to a glass tube, evaporated in a water bath at 45 °C and then reconstituted by adding 600 μl assay buffer and then assayed for T, 11-KT and 17,20β-P. Steroid concentrations are given as nanogram per millilitre.
(e) Data analysis
Data transformations were conducted in order to satisfy the assumptions of parametric analysis. That is, milt volumes and spermatocrit values were log-transformed and percent motility was arcsine-transformed; plasma concentrations of 17,20β-P were log2-transformed while 11-KT and T were logN-transformed. Values in figures and tables are, however, reported from untransformed frequency distributions. Differences in sperm velocity and percent motile cells between dominant and subordinate males were tested by repeated measurements ANOVA, using measurements of sperm velocity and percent motile cells from 15 to 50 s after activation as the within subject variables. We used contrast analysis for post hoc tests following the ANOVA. As plasma steroid concentrations differ between dominants and subordinates, the effect of plasma sex steroids on milt volume and spermatocrit were analysed using a general linear model (GLM) (type I Sum of Squares) after controlling for status, with steroids values as covariates. The effect of plasma sex steroids on sperm velocity and percent motile cells was tested by repeated measurements ANCOVA, where sperm velocity and percent motile cells after activation (15–50 s) were the within subject variable and plasma sex steroid concentrations the covariate. As the correlation between the three different plasma sex steroids were highly positive (T versus 11-KT, r=0.92, T versus 17,20β-P r=0.76, 11-KT versus 17,20β-P r=0.65, all ps<0.0001), we analysed each steroid separately. Following Nakagawa (2004), we did not use Bonferroni or similar adjustments to correct for multiple comparisons, but report all tests probabilities from two-tailed tests. As not all parameters were successfully sampled for all individuals, samples size varies. We used Statistica v. 6.1. (StatSoft, Inc. Tulsa, USA).
3. Results
There was a status dependent change in spermatocrit during the experimental period. That is, compared to pre-trial levels, when there was no difference between the two groups, subordinate males increased while dominant males decreased their spermatocrit during the experimental period (figure 1, Repeated measure ANOVA, F1,46=28.118, p<0.001). There was, on the other hand, no difference between the dominant (0.47 ml±0.43 s.d.) and subordinate (0.32 ml±0.28 s.d.) males in milt volume produced during the experimental period (ANOVA F1,46=1.487, p=0.23).
Figure 1.
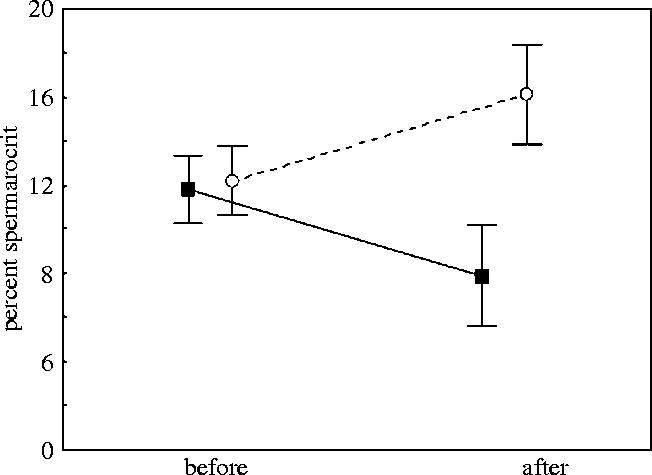
Spermatocrit (%) of ejaculates in subordinate (n=24, open circles) and dominant (n=24, filled squares) males before and after the experimental period. Vertical bars denote 95% confidence intervals.
VCL declined significantly from 15 to 50 s after sperm activation, in samples taken both before and after the experimental period (repeated measurements ANOVA, F4,180=294, p<0.001 and F4,172=359, p<0.001, respectively). Effect of male status on VCL was not found before the experimental period, but after (F1,45=0.14 405, p=0.71, F1,43=5.1638, p=0.028, respectively). A contrast analysis confirmed that there was no significant difference in sperm swimming speed between dominant and subordinate before the experiment at any time (15–50 s after activation, all ns). However, after the experimental period, contrast analysis revealed that subordinate males have significantly higher sperm velocity than dominant males at 15 and 20 s after activation (F1,43=4.15, p=0.048 and F1,43=4.16, p=0.048, respectively). There was, on the other hand, no difference at 30, 40 and 50 s after sperm cell activation (all ns, figure 2). Yet, no significant status specific decline in VCL, either before or after the experimental period, was detected (repeated measurements (VCL 15–50 s×status) ANOVA, F4,180=0.20, p=0.94 and F4,172=1.19, p=0.32, respectively). Despite a significant difference in initial VCL between subordinate and dominant males after the experimental period, there was no significant change in VCL between dominant and subordinate males, measured before and after the experimental period (repeated measurements (VCL before and after×status) ANOVA, F1,44=2.0835, p=0.156, F1,44=1.5531, p=0.219, at respectively, 15 and 20 s after sperm activation, figure 3).
Figure 2.
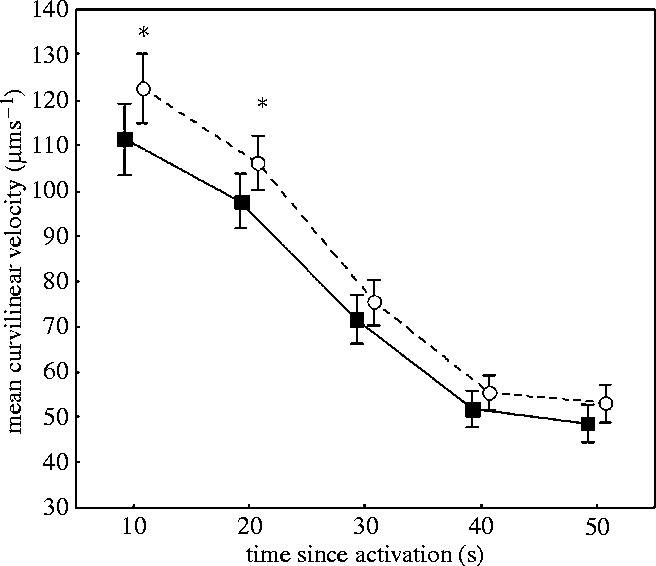
Mean sperm velocity (VCL) after social status was established among subordinate (n=23, open circles) and dominant (n=22, filled squares) males measured at different time (s) after activation. Vertical bars denotes 95% confidence intervals and * denotes significance at 0.05 level.
Figure 3.
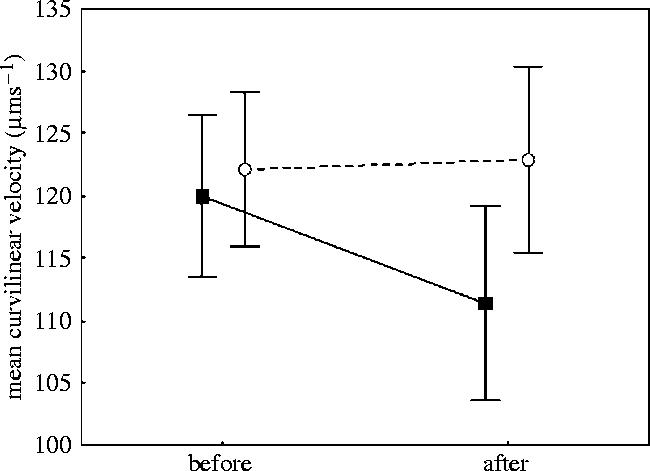
Mean sperm velocity (VCL) before and after the experimental period in subordinates (n=23, open circles) and dominants (n=22, filled squares) males measured at 15 s after activation of sperm cells. Vertical bars denotes 95% confidence intervals.
The percentage of sperm that remained motile in samples taken before and after the experimental period, decreased significantly over time (repeated measurements ANOVA, F4,180=85, p<0.001 and F4,172=140, p<0.001, respectively). Yet, the percentage of motile sperm did not decline at different rates in dominant and subordinate males, either before or after social status was established (Repeated Measurements (percentage of motile 15–50 s×status) ANOVA, F4,180=0.60, p=0.66 and F4,172=0.36, p=0.84, respectively). Nor did status have any effect on percentage of motile sperm, either before or after the experimental period (F1,45=2.9328, p=0.094 and F1,43=0.23 249, p=0.63, respectively, figure 4). However, contrast analyses revealed that after the experimental period, subordinate males had significantly higher frequency of motile cells than dominant males, but only at 20 s post activation (F1,43=5.09, p=0.03).
Figure 4.
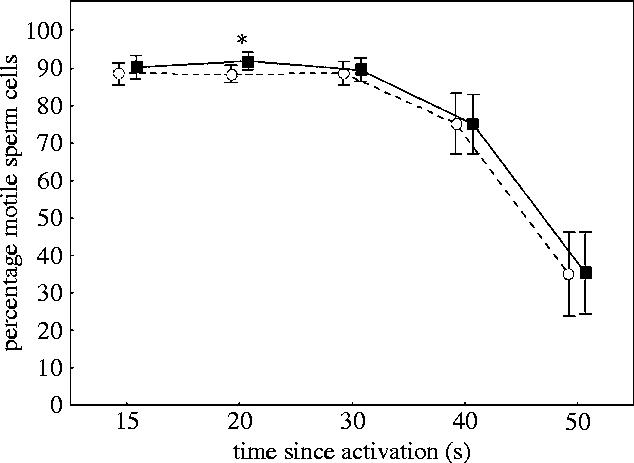
Percent motile sperm cells after social status was established among subordinate (n=23, open circles) and dominant (n=22, filled squares) males measured at different time (s) after activation. Vertical bars denotes 95% confidence intervals and * denotes significance at 0.05 level.
Subordinate males had significantly lower concentrations of the three examined plasma sex steroids (table 1), but plasma sex steroid concentrations did not predict sperm velocity after the experimental period (table 2). Furthermore, plasma sex steroid concentrations did not covary with percent motile cells, milt volume and spermatocrit after controlling for status (table 2).
Table 1.
Dominant and subordinate Arctic charr differed in plasma sex steroid concentrations (ng ml−1) after the experimental period.
| dominant | subordinate | |||||
|---|---|---|---|---|---|---|
| steroid | mean (s.d.) | mean (s.d.) | N | d.f. | F-value | p |
| testosterone | 30.28 (22.1) | 6.04 (5.5) | 46 | 1 | 47.43 | <0.001 |
| 11-ketotestoserone | 60.13 (29.1) | 14.90 (13.5) | 46 | 1 | 38.70 | <0.001 |
| 17,20β-P | 44.04 (25.9) | 16.15 (33.3) | 46 | 1 | 33.01 | <0.001 |
Table 2.
After controlling for status there was no association between plasma sex steroid concentrations (ng ml−1) and frequency of motile sperm cells, sperm velocity (VCL), milt volume and spermatocrit.
| testosterone | 11-ketotestoserone | 17,20β-P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| response variable | F-value | d.f. | p | F-value | d.f. | p | F-value | d.f. | p |
| percent motile cell (15–50 s) | 1.33 | 4, 164 | 0.26 | 1.92 | 4, 164 | 0.11 | 0.49 | 4, 164 | 0.74 |
| VCL (15–50 s) | 1.64 | 4, 164 | 0.17 | 1.08 | 4, 164 | 0.37 | 1.01 | 4, 164 | 0.40 |
| milt volume | 1.54 | 1, 43 | 0.22 | 0.62 | 1, 43 | 0.43 | 2.74 | 1, 43 | 0.11 |
| spermatocrit | 0.06 | 1, 43 | 0.82 | 0.06 | 1, 43 | 0.80 | 0.10 | 1, 43 | 0.75 |
4. Discussion
Our results show that males are capable of rapidly altering ejaculate characteristics in response to changes in social position. Males that become subordinate produce ejaculates with higher sperm cell densities and higher initial sperm velocity than males attaining dominance. Yet, the ejaculate quality seems not to be positively associated with the levels of plasma androgens. Rather, the highest concentrations of plasma sex steroids were found in dominant males, which also had the lowest ejaculate quality.
Subordinate males have higher spermatocrit of ejaculates than dominant males. This is in accordance with results from a previous study in Arctic charr (Liljedal & Folstad 2003) and concur with results from other species (Leach & Montgomerie 2000; Liley et al. 2002; Vladic & Jarvi 2001; delBarco-Trillo & Ferkin 2004, but see Koyama & Kamimura 2000). The difference in spermatocrit between subordinate and dominant males seem to be a response to the social position attained as there is no difference before the experiment started in spermatocrit between those that later became dominant and subordinate. Moreover, as there was no difference between dominant and subordinate males in milt volume produced during the experiment, the tactic specific changes in spermatocrit indicate differences in ejaculate investments. That is, subordinate males increase their ejaculate investment compared to their pre-trial levels, whereas dominant males decrease their investment. The former may be an adaptation to the increased risk of sperm competition, as a subordinate male most likely achieves mating opportunities by sneaking, whereas the latter may be an adaptation to a higher frequency of spawning (i.e. a lower investment in sperm per spawning). Moreover, the higher initial sperm velocity among subordinates may additionally compensate for the disadvantages of spawning in a disfavoured role. As contact with water leads to closure of the micropyle of the egg (Billard 1988), there is likely to be strong selection for high initial sperm velocity (Parker 1993; Parker et al. 1996; Ball & Parker 1996). Differences in sperm velocity may have important implications for fitness (Parker 1990; Birkhead & Pape Møller 1998) and in vitro fertilizations under sperm competition between pairs of Arctic charr have shown that sperm velocity is of major importance for paternity when everything else is held equal (Liljedal 2005). Yet, male Arctic charr occupy different mating roles (Sigurjonsdottir & Gunnarsson 1989) and dominant males may fully compensate from their slower sperm by being in a favoured spawning position, closer to, and in synchrony with the females.
The increased velocity in subordinates, do however, not seem to be traded against longevity as there is no difference between subordinates and dominants in percentage of motile cells 40 and 50 s after activation. In fact, subordinates, rather than dominants, seem to have the highest percentage motile cells, but only 20 s after activation. The proximate explanation for the observed velocity differences may be related to differences in intra-cellular ATP stores (Jeulin & Soufir 1992). This is documented in bluegills, where nest-holding males have lower ATP stores in sperm cells than sneaker males, showing different energetic investment among individuals of different reproductive roles (Burness et al. 2004, but see Burness et al. 2005). Surprisingly, the difference in sperm velocity seems mainly to arise from dominants reducing their sperm velocity compared to their pre-trial levels rather than subordinates increasing their sperm velocity (see figure 3). This may indicate that some of the fish included in the experiment are too small to attain dominance in their natural spawning environment, where individuals approximately 10–15 cm longer normally dominate (own observations). Thus, many of our pre-trial milt samples may be from originally subordinate individuals and adjustments of sperm velocity during the trial should consequently only be evident in individuals changing their pre-trial status, i.e. the individuals becoming dominant.
We found that socially dominant males had higher levels of plasma androgens. This corresponds to the general pattern for fish with alternative mating tactics (Brantley et al. 1993; Borg 1994; Antonopoulou et al. 1999; Schulz & Miura 2002). Eleven-ketotestosterone and Testosterone are androgens known to regulate spermatogenesis in male teleosts (Billard et al. 1982) and 17,20β-P may induce sperm production and are suggested to be involved in regulation of sperm motility in teleost fish (Nagahama 1994; Thomas et al. 1997; Yueh & Chang 1997). However, experimental studies report equivocal result of the effect of androgens on sperm motility and ejaculate characteristics in fish (Lahnsteiner et al. 1996; Clearwater & Crim 1998; Pankhurst & Poortenaar 2000; King & Young 2001; Lim et al. 2004). Our measurements of plasma sex steroid concentrations may not necessarily reflect the intra-testicular steroid concentrations, which are those likely to influence sperm production (Borg 1994). This, together with a relatively short experimental period, may explain why we did not find any relationship between sperm traits and concentrations of plasma sex steroids when social status was controlled for. Additionally, we find no support for androgen mediated reduction of autoimmunity towards individual sperm cells, as dominant males have the lowest sperm velocity despite having the highest levels of immunosuppressive steroids. The formation of sperm cells and their interaction with immunologically active cells is a complicated process controlled by numerous hormones and unknown factors (reviewed in Miura & Miura 2003). For example, as the pH value of semen has been found to be higher in the sperm duct than in the testis, where the sperm remain non-motile, sperm motility may partly be controlled by compounds in the sperm duct (Morisawa & Morisawa 1988). Consequently, the changes in ejaculate characteristics and sperm traits documented in the present study, are unlikely to be controlled by plasma sex steroids alone. Rather, other costly mechanisms giving appropriate pay-offs for a given social position may be at work.
Males from many species develop different tactics to overcome sperm competition (Pilastro et al. 2002; Pizzari et al. 2003; Smith et al. 2003; delBarco-Trillo & Ferkin 2004). When males employ different reproductive tactics, investments in sperm seem to depend on their reproductive role (Burness et al. 2004). We show that the male mating role influences the ejaculate quality, and suggest that this difference is a response to sperm competition risk. That is, male Arctic charr may, to some extent, compensate for the disadvantage of an unfair raffle by rapid adjustments of sperm investments. An increased fertilization return is likely to have generated the evolution of mechanisms allowing rapid changes in both sperm production and velocity. Whether these adjustments are regulated by immunological interactions or simply by energetic requirements is not yet established, but our results hint at the latter.
Acknowledgements
We thank Jacob Lohm, Davnah Urbach and Maria Hansson for assistance during field work. We thank Rüdiger W. Schulz, University of Utrecht, Utrecht, The Netherlands and Alexander P. Scott CEFAS Weymouth Laboratory, Weymouth, UK for kindly providing the 11-KT and 17,20β-P antiserum, respectively. The technical assistant from Tanja L. Hanebrekke with steroid analysis was highly appreciated. We thank Audun Stien for help with the statistics and two anonymous referees for constructive remarks and comments on the manuscript.
References
- Al-Qarawi A.A, Abdel-Rahman H.A, El-Mougy S.A, El-Belely M.S. Use of a new computerized system for evaluation of spermatozoal motility and velocity characteristics in relation to fertility levels in dromedary bulls. Anim. Reprod. Sci. 2002;74:1–9. doi: 10.1016/s0378-4320(02)00163-x. doi:10.1016/S0378-4320(02)00163-X [DOI] [PubMed] [Google Scholar]
- Antonopoulou E, Mayer I, Borg B, Swanson P, Murza I, Christoforov O. Effects of testosterone on gonadotropins, testes, and plasma 17 alpha,2 beta-dihydroxy-4-pregnene-3-one levels in postbreeding mature Atlantic salmon, Salmo salar, male parr. J. Exp. Zool. 1999;284:425–436. doi:10.1002/(SICI)1097-010X(19990901)284:4<425::AID-JEZ9>3.0.CO;2-7 [PubMed] [Google Scholar]
- Ball M.A, Parker G.A. Sperm competition games: external fertilization and ‘adaptive’ infertility. J. Theor. Biol. 1996;180:141–150. doi: 10.1006/jtbi.1996.0090. doi:10.1006/jtbi.1996.0090 [DOI] [PubMed] [Google Scholar]
- Billard R. Artificial insemination and gamete management in fish. Marine Behav. Physiol. 1988;14:3–21. [Google Scholar]
- Billard R, Fostier A, Weil C, Breton B. Endocrine control of spermatogenesis in teleost fish. Can. J. Fish. Aquat. Sci. 1982;39:65–79. [Google Scholar]
- Birkhead T.R, Møller A.P. Academic Press; San Diego: 1998. Sperm competition and sexual selection. [Google Scholar]
- Birkhead T.R, Martinez J.G, Burke T, Froman D.P. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. B. 1999;266:1759–1764. doi: 10.1098/rspb.1999.0843. doi:10.1098/rspb.1999.0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg B. Androgens in teleost fishes. Comp. Biochem. Phys. C—Pharmacol. Toxicol. Endocrinol. 1994;109:219–245. [Google Scholar]
- Brantley R.K, Wingfield J.C, Bass A.H. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fishes. Horm. Behav. 1993;27:332–347. doi: 10.1006/hbeh.1993.1025. doi:10.1006/hbeh.1993.1025 [DOI] [PubMed] [Google Scholar]
- Burness G, Casselman S.J, Schulte-Hostedde A.I, Moyes C.D, Montgomerie R. Sperm swimming speed and energetics vary with sperm competition risk in bluegill (Lepomis macrochirus) Behav. Ecol. Sociobiol. 2004;56:65–70. doi:10.1007/s00265-003-0752-7 [Google Scholar]
- Burness G, Moyes C.D, Montgomerie R. Motility, ATP levels and metabolic enzyme activity of sperm from bluegill (Lepomis macrochirus) Comp. Biochem. Phys. A—Mol. Integr. Phys. 2005;140:11–17. doi: 10.1016/j.cbpb.2004.09.021. doi:10.1016/j.cbpb.2004.09.021 [DOI] [PubMed] [Google Scholar]
- Clearwater S.J, Crim L.W. Gonadotropin releasing hormone-analogue treatment increases sperm motility, seminal plasma pH and sperm production in yellowtail flounder Pleuronectes ferrugineus. Fish Physiol. Biochem. 1998;19:349–357. doi:10.1023/A:1007759620936 [Google Scholar]
- Cutts C.J, Adams C.E, Campbell A. Stability of physiological and behavioural determinants of performance in Arctic char (Salvelinus alpinus) Can. J. Fish. Aquat. Sci. 2001;58:961–968. doi:10.1139/cjfas-58-5-961 [Google Scholar]
- Defraipont M, Fitzgerald G.J, Guderley H. Age-related differences in reproductive tactics in the 3-spined stickleback, Gasterosteus aculeatus. Anim. Behav. 1993;46:961–968. doi:10.1006/anbe.1993.1277 [Google Scholar]
- delBarco-Trillo J, Ferkin M.H. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. doi:10.1038/nature02845 [DOI] [PubMed] [Google Scholar]
- DeWoody J.A, Avise J.C. Genetic perspectives on the natural history of fish mating systems. J. Hered. 2001;92:167–172. doi: 10.1093/jhered/92.2.167. doi:10.1093/jhered/92.2.167 [DOI] [PubMed] [Google Scholar]
- Eberhard W.G.Female control: sexual selection by cryptic female choice1996Princeton University Press; Princeton, NJ [Google Scholar]
- Fabricius E, Gustafson K. Further aquarium observations on the spawning behaviour of the char, Salmo alpinus. Institute of Freshwater Research Report. Drottningholm. 1954;35:58–104. [Google Scholar]
- Folstad I, Karter A.J. Parasites, bright males and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi:10.1086/285346 [Google Scholar]
- Folstad I, Skarstein F. Is male germ line control creating avenues for female choice? Behav. Ecol. 1997;8:109–112. [Google Scholar]
- Frantzen M, Arnesen A.M, Damsgard B, Tveiten H, Johnsen H.K. Effects of photoperiod on sex steroids and gonad maturation in Arctic charr. Aquaculture. 2004;240:561–574. doi:10.1016/j.aquaculture.2004.07.013 [Google Scholar]
- Froman D.P, Feltmann A.J, Rhoads M.L, Kirby J.D. Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus) Biol. Reprod. 1999;61:400–405. doi: 10.1095/biolreprod61.2.400. [DOI] [PubMed] [Google Scholar]
- Gage M.J.G, Macfarlane C.P, Yeates S, Ward R.G, Searle J.B, Parker G.A. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 2004;14:44–47. [PubMed] [Google Scholar]
- Gage M.J.G, Stockley P, Parker G.A. Effects of alternative male mating strategies on characteristics of sperm production in the Atlantic salmon (Salmo salar): Theoretical and empirical investigations. Phil. Trans. Proc. R. Soc. B. 1995;350:391–399. [Google Scholar]
- Gross M.R. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. doi:10.1016/0169-5347(96)81050-0 [DOI] [PubMed] [Google Scholar]
- Grossman C.J. Regulation of the immune-system by sex steroids. Endocr. Rev. 1984;5:435–455. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- Hillgarth N, Ramenofsky M, Wingfield J. Testosterone and sexual selection. Behav. Ecol. 1997;8:108–109. [Google Scholar]
- Hoysak D.J, Liley N.R. Fertilization dynamics in sockeye salmon and a comparison of sperm from alternative male phenotypes. J. Fish Biol. 2001;58:1286–1300. doi:10.1111/j.1095-8649.2001.tb02286.x [Google Scholar]
- Iwamatsu T, Onitake K, Yoshimoto Y, Hiramoto Y. Time sequence of early events in fertilization in the medaka egg. Dev. Growth Diff. 1991;33:479–490. doi: 10.1111/j.1440-169X.1991.00479.x. doi:10.1111/j.1440-169X.1991.00479.x [DOI] [PubMed] [Google Scholar]
- Jeulin C, Soufir J.C. Reversible intracellular ATP changes in intact rat spermatozoa and effects on flagellar sperm movement. Cell Motil. Cytoskel. 1992;21:210–222. doi: 10.1002/cm.970210305. doi:10.1002/cm.970210305 [DOI] [PubMed] [Google Scholar]
- Kime D.E, Ebrahimi M, Nysten K, Roelants I, Rurangwa E, Moore H.D.M, Ollevier F. Use of computer assisted sperm analysis (CASA) for monitoring the effects of pollution on sperm quality of fish; Application to the effects of heavy metals. Aquat. Toxicol. 1996;36:223–237. doi:10.1016/S0166-445X(96)00806-5 [Google Scholar]
- Kime D.E, Van Look K.J.W, McAllister B.G, Huyskens G, Rurangwa E, Ollevier F. Computer-assisted sperm analysis (CASA) as a tool for monitoring sperm quality in fish. Comp. Biochem. Physiol. C—Toxicol. Pharmacol. 2001;130:425–433. doi: 10.1016/s1532-0456(01)00270-8. doi:10.1016/S1532-0456(01)00270-8 [DOI] [PubMed] [Google Scholar]
- King H.R, Young G. Milt production by non-spermiating male Atlantic salmon (Salmo salar) after injection of a commercial gonadotropin releasing hormone analog preparation, 17[alpha]-hydroxyprogesterone or 17[alpha],20[beta]-dihydroxy-4-pregnen-3-one, alone or in combination. Aquaculture. 2001;193:179–195. doi:10.1016/S0044-8486(00)00472-5 [Google Scholar]
- Klein S.L. Hormones and mating system affect sex and species differences in immune function among vertebrates. Behav. Process. 2000;51:149–166. doi: 10.1016/s0376-6357(00)00125-x. doi:10.1016/S0376-6357(00)00125-X [DOI] [PubMed] [Google Scholar]
- Kortet R, Vainikka A, Rantala M.J, Taskinen J. Sperm quality, secondary sexual characters and parasitism in roach (Rutilus rutilus L.) Biol. J. Linnean Soc. 2004;81:111–117. doi:10.1111/j.1095-8312.2004.00275.x [Google Scholar]
- Koyama S, Kamimura S. Influence of social dominance and female odor on the sperm activity of male mice. Physiol. Behav. 2000;71:415–422. doi: 10.1016/s0031-9384(00)00361-9. doi:10.1016/S0031-9384(00)00361-9 [DOI] [PubMed] [Google Scholar]
- Lahnsteiner F, Berger B, Weismann T, Patzner R.A. Motility of spermatozoa of Alburnus alburnus (Cyprinidae) and its relationship to seminal plasma composition and sperm metabolism. Fish Physiol. Biochem. 1996;15:167–179. doi: 10.1007/BF01875596. doi:10.1007/BF01875596 [DOI] [PubMed] [Google Scholar]
- Leach B, Montgomerie R. Sperm characteristics associated with different male reproductive tactics in bluegills (Lepomis macrochirus) Behav. Ecol. Sociobiol. 2000;49:31–37. doi:10.1007/s002650000268 [Google Scholar]
- Levitan D.R. Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. B. 2000;267:531–534. doi: 10.1098/rspb.2000.1032. doi:10.1098/rspb.2000.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley N.R, Tamkee P, Tsai R, Hoysak D.J. Fertilization dynamics in rainbow trout (Oncorhynchus mykiss): effect of male age, social experience, and sperm concentration and motility on in vitro fertilization. Can. J. Fish. Aquat. Sci. 2002;59:144–152. doi:10.1139/f01-202 [Google Scholar]
- Liljedal, S. 2005 Factors influencing sperm production, sperm competition and male fertilization success in the Arctic charr, Salvelinus alpinus Ph.D. Thesis. Norway: University of Tromsø.
- Liljedal S, Folstad I. Milt quality, parasites, and immune function in dominant and subordinate Arctic charr. Can. J. Zool.—Revue Can. De Zool. 2003;81:221–227. doi:10.1139/z02-244 [Google Scholar]
- Lim H.K, Pankhurst N.W, Fitzgibbon Q.P. Effects of slow release gonadotropin releasing hormone analog on milt characteristics and plasma levels of gonadal steroids in greenback flounder, Rhombosolea tapirina. Aquaculture. 2004;240:505–516. doi:10.1016/j.aquaculture.2004.06.017 [Google Scholar]
- Mayer I, Schmitz M, Borg B, Schulz R. Seasonal endocrine changes in male and female Arctic charr (Salvelinus-Alpinus). 1 Plasma-levels of 3 androgens, 17-alpha-hydroxy-20-beta-dihydroprogesterone, and 17-beta-estradiol. Can. J. Zool.—Revue Can. Zool. 1992;70:37–42. [Google Scholar]
- Miura T, Miura C.I. Molecular control mechanisms of fish spermatogenesis. Fish Physiol. Biochem. 2003;28:181–186. doi:10.1023/B:FISH.0000030522.71779.47 [Google Scholar]
- Miura T, Kasugai T, Nagahama Y, Yamauchi K. Acquisition of potential for sperm motility in-vitro in Japanese Eel Anguilla-Japonica. Fish. Sci. 1995;61:533–534. [Google Scholar]
- Miura T, Yamauchi K, Takahashi H, Nagahama Y. Involvement of steroid-hormones in gonadotropin-induced testicular maturation in male Japanese Eel (Anguilla-Japonica) Biomed. Res.-Tokyo. 1991;12:241–248. [Google Scholar]
- Morisawa S, Morisawa M. Induction of potential for sperm motility by bicarbonate and pH in rainbow-trout and chum salmon. J. Exp. Biol. 1988;136:13–22. doi: 10.1242/jeb.136.1.13. [DOI] [PubMed] [Google Scholar]
- Nagahama Y. Endocrine regulation of gametogenesis in fish. Int. J. Dev. Biol. 1994;38:217–229. [PubMed] [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 2004;15:1044–1045. doi:10.1093/beheco/arh107 [Google Scholar]
- Neff B.D, Fu P, Gross M.R. Sperm investment and alternative mating tactics in bluegill sunfish (Lepomis macrochirus) Behav. Ecol. 2003;14:634–641. doi:10.1093/beheco/arg032 [Google Scholar]
- Pankhurst N.W. Hormones and reproductive behavior in male damselfish. Bull. Mar. Sci. 1995;57:569–581. [Google Scholar]
- Pankhurst N.W, Poortenaar C.W. Milt characteristics and plasma levels of gonadal steroids in greenback flounder Rhombosolea tapirina following treatment with exogenous hormones. Mar. Freshwater Behav. Physiol. 2000;33:141–159. [Google Scholar]
- Parker G.A. Sperm competition games—sneaks and extra-pair copulations. Proc. R. Soc. B. 1990;242:127–133. [Google Scholar]
- Parker G.A. Sperm competition games—sperm size and sperm number under adult control. Proc. R. Soc. B. 1993;253:245–254. doi: 10.1098/rspb.1993.0110. [DOI] [PubMed] [Google Scholar]
- Parker G.A, Ball M.A, Stockley P, Gage M.J.G. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. B. 1996;263:1291–1297. [Google Scholar]
- Pilastro A, Scaggiante M, Rasotto M.B. Individual adjustment of sperm expenditure accords with sperm competition theory. Proc. Natl Acad. Sci. USA. 2002;99:9913–9915. doi: 10.1073/pnas.152133499. doi:10.1073/pnas.152133499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Cornwallis C.K, Lovlie H, Jakobsson S, Birkhead T.R. Sophisticated sperm allocation in male fowl. Nature. 2003;426:70–74. doi: 10.1038/nature02004. doi:10.1038/nature02004 [DOI] [PubMed] [Google Scholar]
- Schulz R. Measurement of 5 androgens in the blood of immature and maturing male rainbow-trout, Salmo-gairdneri (Richardson) Steroids. 1985;46:717–726. doi: 10.1016/0039-128x(85)90051-0. doi:10.1016/0039-128X(85)90051-0 [DOI] [PubMed] [Google Scholar]
- Schulz R.W, Miura T. Spermatogenesis and its endocrine regulation. Fish Physiol. Biochem. 2002;26:43–56. doi:10.1023/A:1023303427191 [Google Scholar]
- Scott A.P, Sheldrick E.L, Flint A.P.F. Measurement of 17-alpha,20-beta-dihydroxy-4-pregnen-3-one in plasma of trout (Salmo-gairdneri Richardson)—seasonal-changes and response to salmon pituitary extract. Gen. Comp. Endocrinol. 1982;46:444–451. doi: 10.1016/0016-6480(82)90098-3. doi:10.1016/0016-6480(82)90098-3 [DOI] [PubMed] [Google Scholar]
- Sigurjonsdottir H, Gunnarsson K. Alternative mating tactics of Arctic Charr, Salvelinus-alpinus, in Thingvallavatn, Iceland. Environ. Biol. Fishes. 1989;26:159–176. doi:10.1007/BF00004814 [Google Scholar]
- Skau P.A, Folstad I. Do bacterial infections cause reduced ejaculate quality? A meta-analysis of antibiotic treatment of male infertility. Behav. Ecol. 2003;14:40–47. doi:10.1093/beheco/14.1.40 [Google Scholar]
- Skau P.A, Folstad I. Does immunity regulate ejaculate quality and fertility in humans? Behav. Ecol. 2004;16:410–416. doi: 10.1093/beheco/ari1004 [Google Scholar]
- Slater C.H, Fitzpatrick M.S, Schreck C.B. Androgens and immunocompetence in salmonids: Specific binding in and reduced immunocompetence of salmonid lymphocytes exposed to natural and synthetic androgens. Aquaculture. 1995;136:363–370. doi:10.1016/0044-8486(95)01062-9 [Google Scholar]
- Smith C, Reichard M, Jurajda P. Assessment of sperm competition by European bitterling, Rhodeus sericeus. Behav. Ecol. Sociobiol. 2003;53:206–213. doi:10.1007/s00265-002-0576-x [Google Scholar]
- Stearns S.C, Hoekstra R.F. Oxford University Press; Oxford: 2000. Evolution: an introduction. [Google Scholar]
- Taborsky M. Sperm competition in fish: ‘bourgeois’ males and parasitic spawning. Trends Ecol. Evol. 1998;13:222–227. doi: 10.1016/s0169-5347(97)01318-9. doi:10.1016/S0169-5347(97)01318-9 [DOI] [PubMed] [Google Scholar]
- Thomas P, Breckenridge-Miller D, Detweiler C. Binding characteristics and regulation of the 17,20 beta,21-trihydroxy-4-pregnen-3-one (20 beta-S) receptor on testicular and sperm plasma membranes of spotted sea trout (Cynoscion nebulosus) Fish Physiol. Biochem. 1997;17:109–116. doi:10.1023/A:1007781128677 [Google Scholar]
- Turek P.J, Malkowicz S.B, Tomaszewski J.E, Wein A.J, Peehl D. The role of the Sertoli cell in active immunosuppression in the human testis. Br. J. Urol. 1996;77:891–895. doi: 10.1046/j.1464-410x.1996.00322.x. [DOI] [PubMed] [Google Scholar]
- Uglem I, Galloway T.F, Rosenqvist G, Folstad I. Male dimorphism, sperm traits and immunology in the corkwing wrasse (Symphodus melops L.) Behav. Ecol. Sociobiol. 2001;50:511–518. doi:10.1007/s002650100392 [Google Scholar]
- Vladic T.V, Jarvi T. Sperm quality in the alternative reproductive tactics of Atlantic salmon: the importance of the loaded raffle mechanism. Proc. R. Soc. B. 2001;268:2375–2381. doi: 10.1098/rspb.2001.1768. doi:10.1098/rspb.2001.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh W.S, Chang C.F. 17 alpha,20 beta,21-trihydroxy-4-pregnen-3-one and 17 alpha,20 beta-dihydroxy-4-pregnen-3-one stimulated spermiation in protandrous black porgy, Acanthopagrus schlegeli. Fish Physiol. Biochem. 1997;17:187–193. doi:10.1023/A:1007774628330 [Google Scholar]


