Fig. 1.
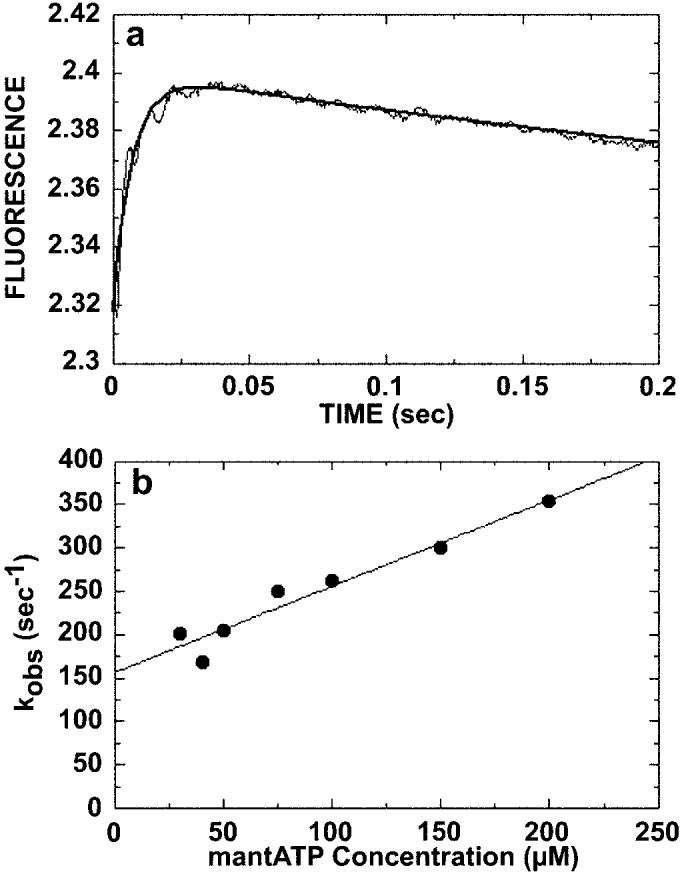
Kinetics of ATP binding. The preformed Mt·K401-4 complex (30 μm tubulin, 10 μm K401-4) was rapidly mixed in the stopped-flow apparatus with varying concentrations of mantATP (20–200 μm). A, a representative stopped-flow record (average of seven individual traces) from an experiment at 20 μm mantATP. An increase in fluorescence was observed, and the smooth line is the fit of the data to a double exponential. The observed rate of the initial fast exponential phase was 135 ± 4.3 s−1. The slow, declining phase at 3.1 ± 0.9 s−1 is too slow to be ATP hydrolysis and is believed to represent an isomerization after initial mantATP binding. B, the observed rate constant of the initial, fast phase increased linearly with increasing mantATP concentration. The data were fit to Equation 1, and the slope provides the apparent second order rate constant for mantATP binding, k+1 = 1 ± 0.1 μm−1 s−1, and the y intercept provides the off-rate, k−1 = 157 ± 11.8 s−1 (Scheme 1).
