Fig. 6.
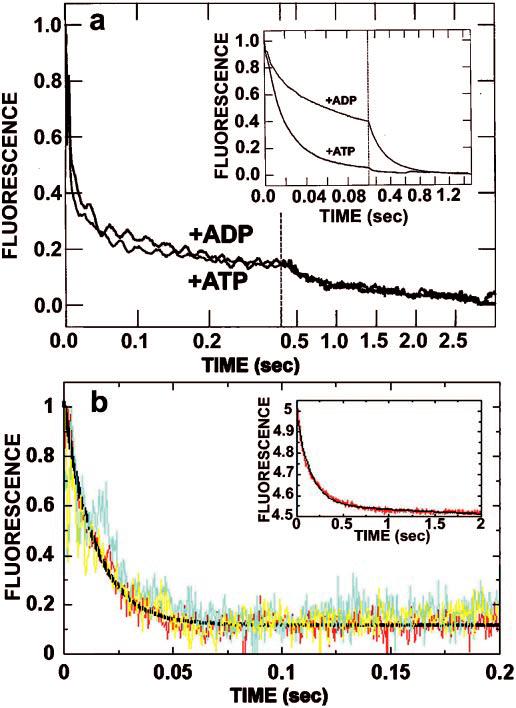
mantADP release in the presence of ATP or ADP. A, kinetics of mantADP release from mutant K401-4 promoted by ATP or ADP. These are representative stopped-flow records (average of six traces) in which the K401-4·mantADP complex (3 μm K401-4, 12 μm mantADP; preformed such that K401-4 contained mantADP bound to both heads) was rapidly mixed with 10 μm microtubules plus either 1 mm MgATP or 1 mm MgADP. It is noteworthy that the mantADP release transients promoted by ADP and ATP are indistinguishable (115.8 ± 4.5 and 107.7 ± 4.0 s−1, respectively). Only 15% of the total amplitude is associated with the second phase. Time domains are 0–400 ms and 400 ms to 3 s. Inset, mantADP release from K401-wt initiated by ATP and ADP. The experiment shown in a was repeated with wild type (3 μm K401 6 μm mantADP). Note that the ADP-promoted kinetic transient shows that approximately 50% of the release amplitude was associated with the initial fast exponential phase (53.6 ± 0.7 s−1), and 50% was associated with the second, much slower exponential phase (4.5 ± 0.02 s−1). Time domains are 0–100 ms and 100 ms to 2 s. B, kinetics of mantADP release from the high affinity nucleotide binding site of K401-4 and K401-wt as a function of ATP or ADP. The Mt·K401-4·mantADP complex was preformed (6 μmm K401-4, 3 μm mantADP, 15 μm microtubules; one mantADP bound per K401-4 dimer) and mixed with either 1 mm MgATP or 1 mm MgADP. Note that the kinetics of mantADP release promoted by ADP and ATP are similar with kobs = 61.1 ± 2.7 s−1 for ATP (yellow) and 54.3 ± 2.5 s−1 for ADP (blue). This experiment was repeated with K401-wt (red), and similar results were observed with 1 mm MgATP, kobs = 73.3 ± 1.7 s−1. Inset, the same experiment repeated with K401-wt with 1 mm MgADP. Note that the rate of ADP release in the presence of ADP is significantly slower for K401-wt; kobs = 6.6 ± 0.1 s−1.
