Abstract
Recent studies have shown that some species of birds have a remarkable degree of control over the sex ratio of offspring they produce. However, the mechanism by which they achieve this feat is unknown. Hormones circulating in the breeding female are particularly sensitive to environmental perturbations, and so could provide a mechanism for her to bias the sex ratio of her offspring in favour of the sex that would derive greatest benefit from the prevailing environmental conditions. Here, we present details of an experiment in which we manipulated levels of testosterone, 17β-oestradiol and corticosterone in breeding female Japanese quail (Coturnix coturnix japonica) using Silastic implants and looked for effects on the sex ratio of offspring produced. Offspring sex ratio in this species was significantly correlated with faecal concentrations of the principal avian stress hormone, corticosterone, and artificially elevated levels of corticosterone resulted in significantly female-biased sex ratios at laying. Varying testosterone and 17β-oestradiol had no effect on sex ratio alone, and faecal levels of these hormones did not vary in response to corticosterone. Our results suggest that corticosterone may be part of the sex-biasing process in birds.
Keywords: maternal effects, hormones, primary sex ratio manipulation, mechanism, Coturnix coturnix japonica
1. Introduction
Recently, there have been a number of reports documenting the ability of birds to facultatively manipulate the sex ratio of their offspring in response to a range of social and environmental factors (e.g. Komdeur et al. 1997; Sheldon et al. 1999; Badyaev et al. 2002). These studies have tended to focus on the adaptive nature of the observed deviations rather than investigating the proximate mechanisms by which they occur, and although several species appear to engage in post-laying manipulation of sex, for example through sex-differential chick mortality (Kilner 1998; Nager et al. 2000), other studies have provided evidence for sex ratio manipulation prior to laying (so-called primary manipulation; e.g. Komdeur et al. 2002) suggesting that the process is under the direct control of the heterogametic (ZW) female (Oddie 1998). However, the underlying mechanism or mechanisms that cause primary avian sex ratio biases remain a mystery.
One possibility is that hormones circulating in the breeding female around the time of sex determination could influence the direction of sex allocation (Pike & Petrie 2003). Concentrations of circulating hormones in birds are remarkably labile, showing not only marked diurnal and seasonal variation but also rapid changes in response to a number of social and environmental variables. It is this plasticity combined with their close association with reproductive physiology that has led to the suggestion that hormones may form an integral part of the sex-biasing mechanism in birds (Krackow 1995; Pike & Petrie 2003). For example, Williams (1999) has reported female-biased secondary sex ratios in zebra finches (Taeniopygia guttata) following 17β-oestradiol injections, and it has been shown that female spotless starlings (Sturnus unicolor) implanted with testosterone produced significantly more sons than females receiving empty control implants (Veiga et al. 2004), although the implants also affected female dominance rank and hence potentially levels of other hormones as well. Similarly, recent work in peafowl (Pavo cristatus) suggests that the proportion of sons produced is positively related to maternal testosterone levels and negatively related to levels of the stress hormone corticosterone (Pike & Petrie 2005a; see also Geiringer 1961 for the effects of corticosterone on sex ratio in rats).
In addition to studies which focus on the role of plasma hormones in determining offspring sex, some interesting relationships have been found between the sex of avian eggs and maternally derived egg-yolk hormones, which appear to reflect the hormonal state of the breeding female during egg production (e.g. Hayward & Wingfield 2004). For example, peafowl allocate hormones differentially to their eggs, with those containing male embryos receiving significantly higher yolk levels of androgens than female eggs, which in turn had significantly elevated concentrations of 17β-oestradiol (Petrie et al. 2001; but see Pilz et al. 2005), and female biases in the sex ratio are associated with elevated yolk corticosterone levels (Pike & Petrie 2005b). However, from these studies it is impossible to tell whether the observed relationship between sex and hormones was one of the cause or effect, or whether hormone levels varied in response to some third variable. The only way to test this explicitly is to manipulate hormone levels experimentally and look at the effects on offspring sex.
In the experiment presented here, we manipulated circulating levels of testosterone, 17β-oestradiol and corticosterone in breeding female Japanese quail (Coturnix coturnix japonica) using Silastic implants and looked for effects on the sex ratio of offspring they produced.
2. Material and methods
The Japanese quail used in this experiment were bred at Newcastle University, UK, and at the start of the experiment were approximately seven weeks old and sexually experienced. Male and female birds were randomly paired (n=45 pairs) and these pairs further subdivided into six groups. They were housed in individual wire mesh cages, which occupied two rooms, and were allowed to acclimatize for 14 days before starting the experiment. All birds were kept on a 14 L : 10 D photoperiod in a temperature-controlled environment (21±1 °C), and received food (pheasant breeder feed), oyster-shell grit, fresh fruit and water ad libitum.
The experiment was divided into two phases. During the first phase, breeding females received no exogenous hormones or anti-hormones, while during the second phase hormone levels were manipulated (up or down) in the same females (see below). We did not include a post-treatment control due to the unknown long-term effects of hormone treatment on birds. During each phase all the eggs laid were collected for 10 days, incubated for 5 days and any embryonic tissue removed and genetically sexed. Each pair thus acted as their own control, so that if one female was predisposed to bias the sex ratio of offspring produced this would not affect the results obtained during hormone treatment.
Hormone levels were manipulated using Silastic implants, which were individually implanted, under anaesthesia (four parts oxygen, two part isoflurane delivered through a face mask), subcutaneously over the left breast. The whole procedure took less than 5 min and birds were allowed to recover for 1–2 h before returning to their cage and a further 22 h before starting the experimental phase. Filled implants contained approximately 2.4 mg of either testosterone (n=8 females), 17β-oestradiol (n=8), fadrazole (a potent aromataze inhibitor, to reduce circulating levels of 17β-oestradiol; n=8), corticosterone (n=8) or metyrapone (a corticosterone-synthesis inhibitor; n=8). Control females received an identical but empty implant. Each implant was pierced once with a 25-gauge needle prior to implantation to facilitate the initial movement of its contents into the blood and changes in hormone levels gauged from levels of hormones excreted in the faeces. Short-term hormone fluctuations, which can be a problem in interpreting plasma hormone levels, are buffered over time in faeces and several studies have shown that faecal hormones correlate well with plasma hormones in a range of different bird species (Bishop & Hall 1991; Cockrem & Rounce 1994; Schwabl 1996; Ludders et al. 2001). Furthermore, faecal samples can be collected non-invasively, causing minimal stress to the birds and so minimizing perturbations in hormone (and particularly corticosterone) levels. Our manipulations significantly raised or lowered faecal (and hence presumably plasma) concentrations of steroids in the predicted directions (figure 1) and in each case, the measured level was within the range previously established for this population (mean±s.d. (range)): testosterone 4.78±2.75 (0.9–9.1) μg l−1, 17β-oestradiol 1.55±0.84 (0.1–4.0) μg l−1 and corticosterone 59.43±15.61 (27.9–107.5) μg l−1.
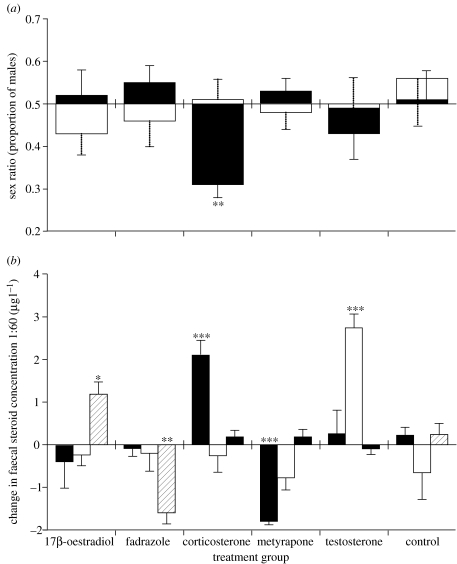
Figure 1.
Offspring sex ratios in relation to plasma hormone levels. (a) Group sex ratios produced during control (white) and hormone treatments (black). (b) Mean (+s.e.) changes in faecal concentrations of corticosterone (/10, black), testosterone (white) and 17β-oestradiol (hatched) from individual females between control and hormone treatment. Corticosterone was significantly elevated during treatment with corticosterone, and significantly lowered by metyrapone (ANOVA between groups with Tukey's pairwise comparisons: F5,39=11.02, p<0.001). 17β-oestradiol and fadrazole significantly raised and lowered 17β-oestradiol concentrations, respectively (F5,39=20.80, p<0.001), while testosterone treatment significantly raised faecal testosterone levels (F5,39=13.82, p<0.001). *p<0.05, **p<0.01, ***p<0.001.
(a) Molecular sexing
Following Chelex extraction of genomic DNA from approximately 0.1 g embryonic tissue, the samples were sexed using the polymerase chain reaction (PCR) to amplify part of the W-linked avian CHD gene (CHD-W) in females, and its non-W-linked homologue (CHD-Z) in both sexes. PCR primers 2718R and 2550F (Fridolfsson & Ellegren 1999) were used to amplify introns of variable length in the two genes from conserved exonic regions. Products were run on a 2% agarose gel and visualized with ethidium bromide. The presence of either one (CHD-Z; males) or two distinct bands (CHD-Z and CHD-W; females) were used to determine sex. All eggs that developed a visible embryo were successfully sexed and the sexing was performed blind and in a random order.
(b) Faecal hormone analysis
Throughout the experiment, faecal samples were collected daily from each female by allowing her to defecate on a sheet of waterproof paper. The samples were then left for 24 h on the paper to dry before collection and storage at −20 °C until extraction. Birds show strong diurnal cyclicity in hormone levels and so all samples were collected at the same time of day and within 1 h of each other (1500–1600 h). Samples collected from an individual female during each experimental phase were pooled for extraction following the method of Bishop & Hall (1991) and frozen at −20 °C until testosterone, 17β-oestradiol and corticosterone levels could be quantified using commercially available enzyme immunoassay kits (Immunodiagnostic Systems Ltd, Bolden, Tyne & Wear). Mean intra-assay coefficients of variation were 2.9, 4.6 and 5.7%, and assay sensitivities were 6, 4.6 and 230 pg ml−1 for testosterone, 17β-oestradiol and corticosterone, respectively. Cross-reactivity (%) for each hormone was as follows: testosterone (dihydrotestosterone: 0.86, androstenedione: 0.89), 17β-oestradiol (estrone: 0.17, estriol: 0.11) and corticosterone (progesterone: 0.05, cortisol: 0.02, testosterone and 17β-oestradiol: <0.01; manufacturer's insert).
(c) Statistical analysis
Sex ratio (number of males/number of sexed eggs) was analysed at the clutch level by fitting a generalized linear mixed model using the GLIMMIX macro (binomial error distribution, logit link function) in SAS v. 8 (Littell et al. 1996). Repeated measures analyses were used to compare changes in sex ratio between phases of the experiment, and in all analyses room was included as a random factor. The GLIMMIX macro automatically adjusts for overdispersion (Littell et al. 1996). Where multiple pairwise comparisons were conducted, the stated p-values have been sequentially Bonferroni corrected (Rice 1989) with α=0.10 (Chandler 1995). All statistical tests are two-tailed and the level of significance set at 5%.
3. Results
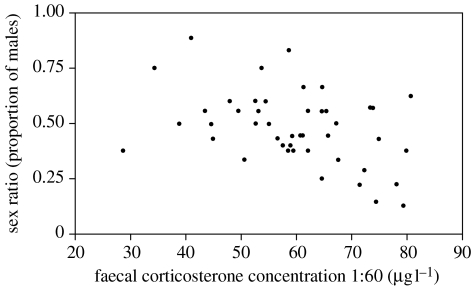
During phase one of the experiment, the sex ratios produced across groups did not differ significantly from parity (F5,38=0.46, p=0.8; figure 1), although individual clutch sex ratios were significantly predicted by a female's faecal corticosterone levels (F1,38=10.27, p=0.003; figure 2). Faecal levels of testosterone and 17β-oestradiol, maternal body condition and paternal tarsus length were unrelated to sex ratio (all p>0.05).
Figure 2.
Relationship between the faecal corticosterone concentration of laying hens and their clutch sex ratio during the control phase (phase one).
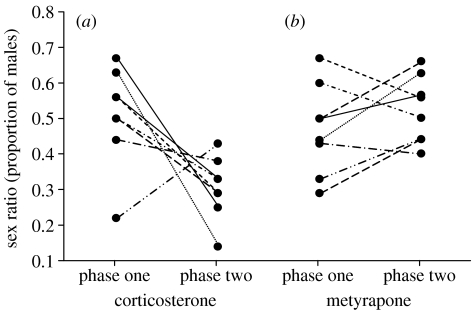
During phase two, while females were undergoing hormone manipulation, the sex ratio differed significantly across groups (F5,38=3.33, p=0.014; figure 1). Specifically, corticosterone-treated quail showed a significant decline in sex ratio between phases (F1,13=11.77, p=0.005, with seven out of eight females in this group showing a decline in sex ratio; figure 3a) and their sex ratio during treatment differed significantly from parity (F1,14=37.82, p<0.001). The sex ratios produced by corticosterone-treated quail were also significantly more female-biased than those of the other groups (all Bonferroni adjusted p<0.05), although there was no evidence that progressively more or less daughters were produced over the 10 days of treatment (F1,13=1.72, p=0.2). Metyrapone (see figure 3b), testosterone, 17β-oestradiol and fadrazole implants caused no significant sex ratio change between phases (all p>0.05). There was no evidence that maternal condition (F5,38=1.39, p=0.251) or paternal tarsus length (F5,38=0.69, p=0.6) differed between treatment groups.
Figure 3.
Individual changes in the sex ratio of (a) corticosterone- and (b) metyrapone-treated females between phase one (control) and phase two (hormone treatment) of the experiment.
No embryonic material could be extracted from infertile eggs or eggs where a visible embryo failed to develop, and as a result not all of the eggs laid could be successfully sexed. However, the proportion of infertile eggs produced by the corticosterone-treated group did not differ significantly between the first (no bias) and second (female biased) phases (F1,14=1.32, p=0.3). This suggests that the sex ratio biases were present at laying, and not the result of early sex-differential embryo mortality. Similarly, there was no significant difference in the number of laying sequence gaps (a day or more during which an egg was not laid) between corticosterone-treated quail and control (sign test: n=8 pairs, p=0.6).
4. Discussion
The results of the experiment presented here provide strong evidence for a causal role of corticosterone in the sex manipulating process in birds. During the unmanipulated control phase, there was a significant relationship between an individual female's clutch sex ratio and her faecal levels of corticosterone around the time of egg production. Moreover, artificially elevating corticosterone levels via Silastic implants caused females to produce significantly female-biased offspring sex ratios, while decreasing circulating corticosterone (by administering the corticosterone-synthesis inhibitor metyrapone) caused females to produce slightly more sons than during control, although this result was not significant. Raising or lowering levels of other hormones, including testosterone and 17β-oestradiol, appeared to have no effect on clutch sex ratios, as these remained similar to those produced during the control phase. Our experimental design controlled for individual maternal and paternal differences in sex ratio by comparing eggs laid by the same females during control and treatment phases and it is unlikely that temporal changes in sex ratio could contribute to the changes in sex ratio observed in the corticosterone-treated group as none of the other groups (including the control group) showed any similar trends.
Corticosterone is the major avian glucocorticoid released in response to stress and may allow breeding females to bias the sex ratio of their offspring in response to environmental conditions around the time of egg production. For example, corticosterone is commonly found to be raised in the plasma of birds in poor body condition (e.g. Schoech et al. 1997; Kitaysky et al. 1999) and poor condition is associated with sex ratio biases in a variety of species (e.g. Nager et al. 1999; Kalmbach et al. 2001). The skews in sex ratio we observed during corticosterone treatment were not absolute since every clutch contained at least one male, and so it is possible that corticosterone concentrations did not deviate far enough from base levels to induce particularly large sex ratio skews. However, whether the effect of corticosterone on sex ratio is dose-dependent is not known and certainly warrants further investigation. Another possibility is that corticosterone acts in conjunction with other, as yet unidentified hormones. For example, corticosterone has been shown to have inhibitory effects on reproductive processes, including the production of testosterone (Tokarz 1987; DeNardo & Sinervo 1994; Wingfield et al. 1994), although there was no evidence that levels of testosterone varied significantly during treatment with corticosterone. Similarly, we could find no evidence that the administration of exogenous testosterone raised (Ketterson et al. 1991) or lowered (Pe'czely 1979) corticosterone levels, although the interactions between corticosterone and testosterone are complex and could be obscured by other factors (e.g. Wingfield et al. 1992), and there was no evidence that male-biased sex ratios can be induced by elevating testosterone levels (cf. Veiga et al. 2004). Corticosterone may also vary with concentrations of its precursor, progesterone. Although we did not measure progesterone in this study, recent work suggests that progesterone injections can bias chicken Gallus gallus sex ratios towards daughters (Correa et al. 2005), and so we cannot rule out the possibility that the effects of corticosterone on sex ratio were mediated indirectly by covarying levels of progesterone. Further work is needed to disentangle the relative effects of these two hormones.
Although we could not sex infertile eggs or eggs in which a visible embryo failed to develop, our results suggest that sex-biased infertility or early embryo mortality were not responsible for the observed biases and so the sex ratios analysed probably provide a good approximation of the primary sex ratio. This in turn has implications for the mechanism of manipulation. Avian oocytes undergo the first meiotic division in the ovary a few hours before ovulation (Romanoff & Romanoff 1949). This division consigns one of the sex chromosomes to the polar body, effectively determining the sex of the egg. If the ovulated egg is fertilized, it undergoes the second meiotic division and develops into a male or female embryo (Romanoff & Romanoff 1949). Certain pre-laying mechanisms of sex ratio manipulation such as selective abortion of ‘wrong’ sex follicles would induce frequent laying sequence gaps and delay breeding in species laying large clutches, such as quail (Pike & Petrie 2003). However, this mechanism appears unlikely since the proportion of laying gaps did not differ significantly between phases in the corticosterone-treated group. Instead, it is possible that high circulating levels of corticosterone either influence sex-chromosome segregation at the first meiotic division, or bias which immature follicle undergoes rapid maturation, one destined to become male or female (reviewed in Pike & Petrie 2003), and thus form part of the sex-biasing mechanism in birds. Corticosterone could potentially be used to bias sex ratios ‘on demand’ and so facilitate further investigation into the precise mechanism by which sex manipulation occurs, as well as providing insight into the costs and limitations of manipulation.
Acknowledgments
We are grateful to BBSRC for financial support and for the helpful comments of two anonymous referees. The experiment described here was performed under Home Office licence and conforms to the Study of Animal Behaviour/Animal Behaviour Society's Guidelines for the use of animals in Research.
References
- Badyaev A.V, Hill G.E, Beck M.L, Dervan A.A, Duckworth R.A, McGraw K.J, Nolan P.M, Whittingham L.A. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002;295:316–318. doi: 10.1126/science.1066651. 10.1126/science.1066651 [DOI] [PubMed] [Google Scholar]
- Bishop C.M, Hall M.R. Non-invasive monitoring of avian reproduction by simplified faecal steroid analysis. J. Zool. 1991;224:649–668. [Google Scholar]
- Chandler C.R. Practical considerations in the use of simultaneous inference for multiple tests. Anim. Behav. 1995;49:524–527. 10.1006/anbe.1995.0069 [Google Scholar]
- Cockrem J.F, Rounce J.R. Faecal measurements of oestradiol and testosterone allow the non-invasive estimation of plasma steroid concentrations in the domestic fowl. Br. Poult. Sci. 1994;35:433–443. doi: 10.1080/00071669408417708. [DOI] [PubMed] [Google Scholar]
- Correa S.M, Adkins-Regan E, Johnson P.A. High progesterone during avian meiosis biases sex ratios towards females. Biol. Lett. 2005;1:215–218. doi: 10.1098/rsbl.2004.0283. 10.1098/rsbl.2004.0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo D.F, Sinervo B. Effects of steroid-hormone interaction on activity and home-range size of male lizards. Horm. Behav. 1994;28:273–287. doi: 10.1006/hbeh.1994.1023. 10.1006/hbeh.1994.1023 [DOI] [PubMed] [Google Scholar]
- Fridolfsson A.K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. [Google Scholar]
- Geiringer E. Effect of ACTH on sex ratio in the albino rat. Proc. Soc. Exp. Biol. Med. 1961;106:752–754. doi: 10.3181/00379727-106-26464. [DOI] [PubMed] [Google Scholar]
- Hayward L.S, Wingfield J.C. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen. Comp. Endocrinol. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalmbach E, Nager R.G, Griffiths R, Furness R.W. Increased reproductive effort results in male-biased offspring sex ratio: an experimental study in a species with reversed sexual size dimorphism. Proc. R. Soc. B. 2001;268:2175–2179. doi: 10.1098/rspb.2001.1793. 10.1098/rspb.2001.1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson E.D, Nolan V, Wolf L, Ziegenfus C, Dufty A.M, Ball G.F, Johnsen T.S. Testosterone and avian life histories—the effect of experimentally elevated testosterone on corticosterone and body-mass in dark-eyed juncos. Horm. Behav. 1991;25:489–503. doi: 10.1016/0018-506x(91)90016-b. 10.1016/0018-506X(91)90016-B [DOI] [PubMed] [Google Scholar]
- Kilner R. Primary and secondary sex ratio manipulation by zebra finches. Anim. Behav. 1998;56:155–164. doi: 10.1006/anbe.1998.0775. 10.1006/anbe.1998.0775 [DOI] [PubMed] [Google Scholar]
- Kitaysky A.S, Piatt J.F, Wingfield J.C, Romano M. The adrenocortical stress-response of black-legged Kittiwake chicks in relation to dietary restrictions. J. Comp. Physiol. B. 1999;169:303–310. 10.1007/s003600050225 [Google Scholar]
- Komdeur J, Daan S, Tinbergen J, Mateman C. Extreme adaptive modification of the sex ratio of the Seychelles warbler's eggs. Nature. 1997;385:522–525. 10.1038/385522a0 [Google Scholar]
- Komdeur J, Magrath M.J.L, Krackow S. Pre-ovulation control of hatchling sex ratio in the Seychelles warbler. Proc. R. Soc. B. 2002;269:1067–1072. doi: 10.1098/rspb.2002.1965. 10.1098/rspb.2002.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krackow S. Potential mechanisms for sex ratio adjustment in mammals and birds. Biol. Rev. 1995;70:225–241. doi: 10.1111/j.1469-185x.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.S. SAS Institute; North Carolina: 1996. SAS system for mixed models. [Google Scholar]
- Ludders J.W, Langenberg J.A, Czekala N.M, Erb H.N. Fecal corticosterone reflects serum corticosterone in Florida sandhill cranes. J. Wildl. Dis. 2001;37:646–652. doi: 10.7589/0090-3558-37.3.646. [DOI] [PubMed] [Google Scholar]
- Nager R.G, Monaghan P, Griffiths R, Houston D.C, Dawson R. Experimental demonstration that offspring sex ratio varies with maternal condition. Proc. Natl Acad. Sci. USA. 1999;96:570–573. doi: 10.1073/pnas.96.2.570. 10.1073/pnas.96.2.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nager R.G, Monaghan P, Houstan D.C, Genovart M. Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus) Behav. Ecol. Sociobiol. 2000;48:452–457. 10.1007/s002650000262 [Google Scholar]
- Oddie K. Sex discrimination before birth. Trends Ecol. Evol. 1998;13:130–131. doi: 10.1016/s0169-5347(97)01320-7. 10.1016/S0169-5347(97)01320-7 [DOI] [PubMed] [Google Scholar]
- Pe'czely P. Effect of testosterone and thyroxine on corticosterone and transcortine plasma levels in different bird species. Acta Physiol. Acad. Sci. Hung. 1979;53:9–15. [PubMed] [Google Scholar]
- Petrie M, Schwabl H, Brande-Lavridsen N, Burke T. Sex differences in avian yolk hormone levels. Nature. 2001;412:489. doi: 10.1038/35087652. 10.1038/35087652 [DOI] [PubMed] [Google Scholar]
- Pike T.W, Petrie M. Potential mechanisms of avian sex manipulation. Biol. Rev. 2003;78:553–574. doi: 10.1017/s1464793103006146. 10.1017/S1464793103006146 [DOI] [PubMed] [Google Scholar]
- Pike T.W, Petrie M. Maternal body condition and plasma hormones affect offspring sex ratio in peafowl. Anim. Behav. 2005a;70:745–751. 10.1016/j.anbehav.2004.12.020 [Google Scholar]
- Pike T.W, Petrie M. Offspring sex ratio is related to paternal train elaboration and yolk corticosterone in peafowl. Biol. Lett. 2005b;1:204–207. doi: 10.1098/rsbl.2005.0295. 10.1098/rsbl.2005.0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz K.M, Adkins-Regan E, Schwabl H. No sex difference in yolk steroid concentrations of avian eggs at laying. Biol. Lett. 2005;1:318–321. doi: 10.1098/rsbl.2005.0321. 10.1098/rsbl.2005.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Romanoff A.L, Romanoff A.J. Wiley; New York: 1949. The avian egg. [Google Scholar]
- Schoech S.J, Mumme R.L, Wingfield J.C. Corticosterone, reproductive status and body mass in a cooperative breeder, the Florida scrub-jay (Aphelocoma coerulescens) Physiol. Zool. 1997;70:68–73. doi: 10.1086/639545. [DOI] [PubMed] [Google Scholar]
- Schwabl H. Environment modifies the testosterone levels of a female bird and her eggs. J. Exp. Zool. 1996;276:157–163. doi: 10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N. 10.1002/(SICI)1097-010X(19961001)276:2%3C157::AID-JEZ9%3E3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Andersson S, Griffith S.C, Örnborg J, Sendecka J. Ultraviolet colour variation influences blue tit sex ratios. Nature. 1999;402:874–877. 10.1038/47239 [Google Scholar]
- Tokarz R.R. Effects of corticosterone treatment on male aggressive behavior in a lizard (Anolis sagrei) Horm. Behav. 1987;21:358–370. doi: 10.1016/0018-506x(87)90020-1. 10.1016/0018-506X(87)90020-1 [DOI] [PubMed] [Google Scholar]
- Veiga J.P, Viñuela J, Cordero P.J, Aparicio J.M, Polo V. Experimentally increased testosterone affects social rank and primary sex ratio in the spotless starling. Horm. Behav. 2004;46:47–53. doi: 10.1016/j.yhbeh.2004.01.007. 10.1016/j.yhbeh.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Williams T.D. Parental and first generation effects of exogeneous 17β-oestradiol on reproductive performance of female zebra finches (Taeniopygia guttata) Horm. Behav. 1999;35:135–143. doi: 10.1006/hbeh.1998.1506. 10.1006/hbeh.1998.1506 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Vleck C.M, Moore M.C. Seasonal changes of the adrenocortical response to stress in birds of the Sonoran desert. J. Exp. Zool. 1992;264:419–428. doi: 10.1002/jez.1402640407. 10.1002/jez.1402640407 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Suydam R, Hunt K. The adrenocortical responses to stress in snow buntings (Plectrophenax nivalis) and Lapland longspurs (Calcarius lapponicus) at Barrow Alaska. Comp. Biochem. Physiol. B. 1994;108:299–306. [Google Scholar]