Figure 7.
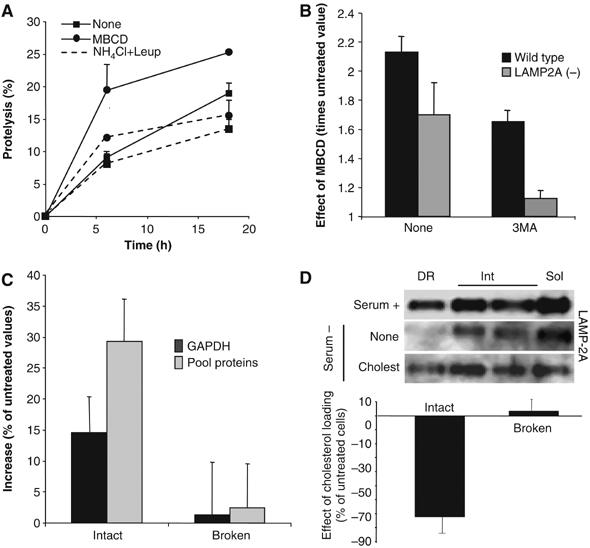
Consequences of changes in the lysosomal cholesterol content on CMA activity. (A) Degradation of long-lived proteins in mouse fibroblasts (NIH3T3 cells) treated or not with 25 mM MBCD. Where indicated, 15 mM NH4Cl/100 μM leupeptin (NH4Cl+Leup) were added into the medium. Values are the mean+s.e. of three experiments with triplicate samples. (B) Effect of MBCD on total rates of protein degradation, measured as in (A), in wild-type mouse fibroblasts and in fibroblasts stably RNA interfered for LAMP-2A (LAMP-2A(−)). The ratio of degradation in MBCD-treated and untreated cells is shown for cells maintained with or without 10 mM 3-methyladenine (3MA). (C) Proteolysis of [14C]GAPDH or a pool of [3H]labeled cytosolic proteins (pool proteins) by isolated rat liver lysosomes, treated or not with MBCD. Broken: lysosomes disrupted by a hypotonic shock before the incubation with the radiolabeled proteins. Values are expressed as percentage of the degradation by untreated lysosomes and are the mean+s.e. of three experiments with triplicate samples. (D) Lysosomal–mitochondrial fractions were isolated from mouse fibroblasts untreated or loaded with cholesterol (50 μM). Top: Distribution of LAMP-2A after Triton X-114 extraction and sucrose density gradient centrifugation. Bottom: Ability of intact or disrupted lysosomal–mitochondrial fractions to proteolize [14C]GAPDH. Values are expressed as percentage of the degradation in fractions from untreated cells and are the mean±s.e. of triplicate samples.
