Figure 3.
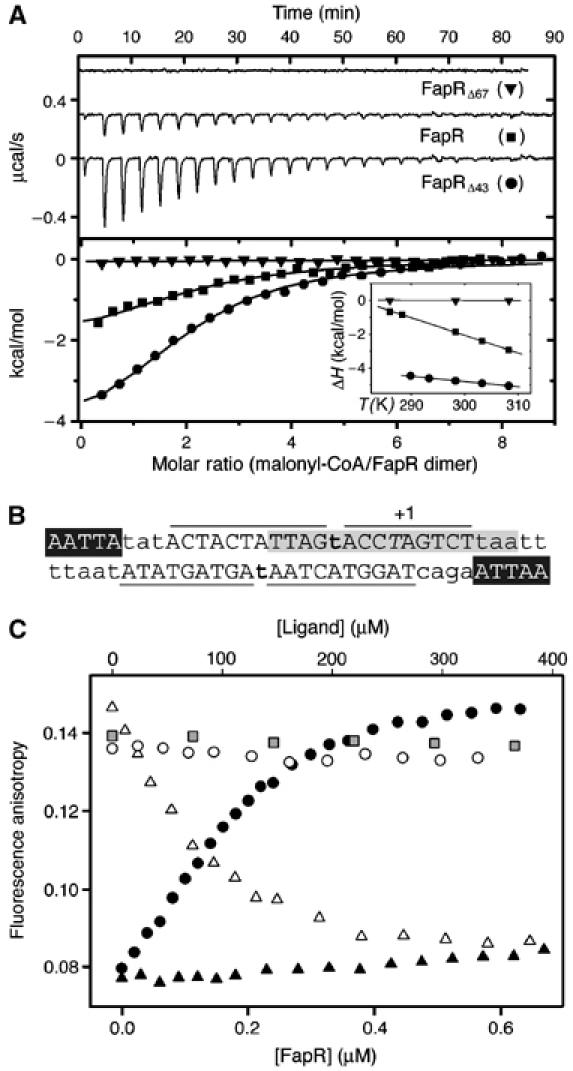
Malonyl-CoA binds to the C-terminal domain of FapR and modulates DNA-binding activity. (A) Isothermal calorimetric titrations of FapR with malonyl-CoA. The top panel shows the raw heat signal for 10 μl injections of 240 μM malonyl-CoA into solutions of 16 μM FapR, 20 μM FapRΔ43, and 20 μM FapRΔ67, respectively, at 298 K (curves have been offset by 0.3 μcal/s for clarity). The bottom panel shows the transition curves of the three proteins (FapR, Kd=2.4±0.2 μM; FapRΔ43, Kd=7.1±0.9 μM). The inset shows the enthalpy changes for the above reactions as a function of temperature (FapR, ΔH°=−1.8±0.2 kcal mol−1, ΔCp=−103.0±0.3 cal mol−1 K−1; FapRΔ43, ΔH°=−4.8±0.3 kcal mol−1, ΔCp=−31.9±1.1 cal mol−1 K−1). FapRΔ67 displays a flat binding isotherm at all tested temperatures between 288 and 308 K. (B) PfapR operator region. Capital letters indicate bases protected by FapR from DNAseI digestion; lines delimitate protected regions. Bold letters show hypersensitive spots. Italic T indicates the transcription start base. The 17 bp inverted repeat conserved in all operators of the fap regulon (Schujman et al, 2003) is shown in gray and white letters indicate a 5 bp inverted repeat separated by 23 nt. (C) Fluorescence anisotropy changes on addition of FapRWT to 9.5 nM 34 bp F-dsDNA (black circles); on addition of FapRWT to a mixture of 9.5 nM 34 bp F-dsDNA and 307.7 μM malonyl-CoA (black triangles); and on addition of malonyl-CoA (white triangles), acetyl-CoA (white circles) or malonic acid (gray squares) to the previously formed F-dsDNA/FapRWT complex.
