Figure 8.
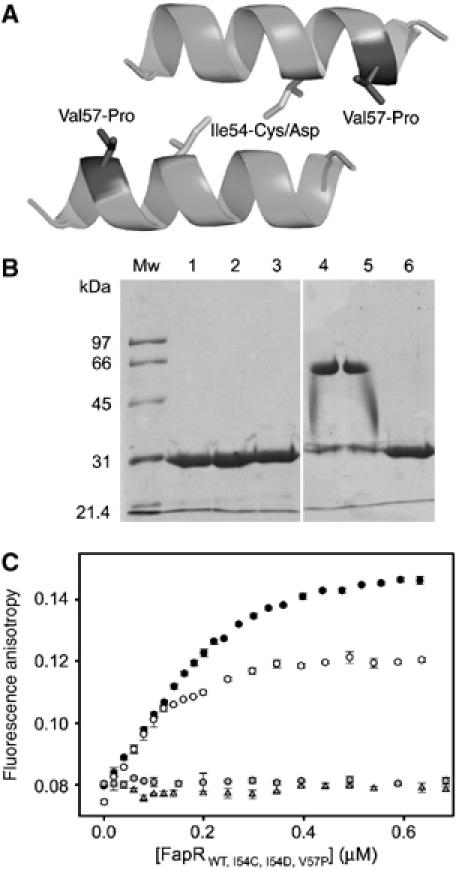
Mutational analysis of linker helix dimerization. (A) Helix–helix interaction as observed in crystal packing contacts of the FapRΔ43–malonyl-CoA complex. The amino-acid positions mutated are indicated. (B) SDS–PAGE of purified FapR (lanes 1–3) and FapRI54C (lanes 4–6) under oxidative (lanes 1 and 4), nonoxidative (lanes 2 and 5) and reducing (lanes 3 and 6) conditions. Both proteins behave as a homodimer in solution, as indicated by gel filtration chromatography (data not shown). (C) Fluorescence anisotropy changes on addition of wild-type FapR (black circles), FapRI54C (white circles), FapRI54D (gray triangles) and FapRV57P (gray circles) to 9.5 nM 34 bp F-dsDNA. As for the wild-type protein, the FapRI54C–DNA complex is dissociated upon addition of malonyl-CoA (data not shown).
