Table 1.
Data collection, phasing and refinement statistics
| Data set | SeMet-labeled FapR | FapRΔ67 | FapRΔ43–malonyl-CoA | ||
|---|---|---|---|---|---|
| Data collection | |||||
| Resolution (Å)a | 40–3.5 (3.69–3.5) | 60–2.5 (2.64–2.5) | 63.2–3.1 (3.27–3.1) | ||
| Wavelength (Å) | 0.9791 | 0.9793 | 0.9755 | 1.072 | 0.9794 |
| Measured reflections | 22 839 | 22 837 | 23 045 | 74 467 | 87 334 |
| Multiplicitya | 5.8 (5.8) | 5.8 (5.7) | 5.8 (5.8) | 5.0 (4.3) | 6.9 (7.2) |
| Completeness (%)a | 99.4 (99.4) | 99.5 (99.7) | 99.3 (99.2) | 80.5 (50.0) | 100 (100) |
| Rsym (%)a,b | 9.3 (27.5) | 9.7 (32.6) | 11.5 (40.3) | 8.3 (28.7) | 7.9 (31.0) |
| 〈I/σ〉a | 13.5 (4.9) | 12.8 (4.2) | 11.3 (3.3) | 13.7 (4.8) | 19.3 (6.0) |
| Refinement | |||||
| Resolution (Å) | 30–2.5 | 63.2–3.1 | |||
| Rcryst c (No. refs) | 0.221 (14035) | 0.187 (11604) | |||
| Rfree c (No. refs) | 0.267 (790) | 0.227 (941) | |||
| R.m.s. bonds (Å) | 0.019 | 0.02 | |||
| R.m.s. angles (deg) | 1.72 | 2.14 | |||
| Protein atoms | 2964 | 2238 | |||
| Water molecules | 8 | 10 | |||
| Ligand atoms | — | 64 | |||
| aValues in parentheses apply to the high resolution shell. | |||||
b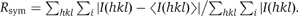
| |||||
c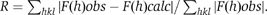
| |||||
| Rcryst and Rfree were calculated from the working and test reflection sets, respectively. | |||||
