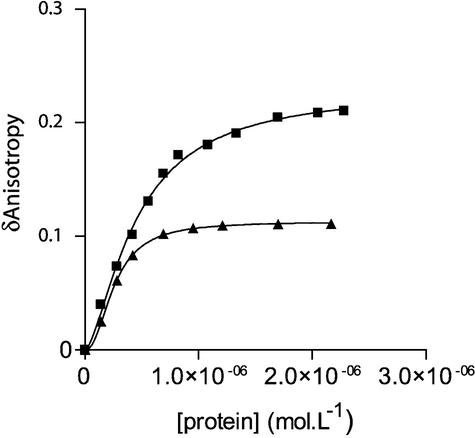
Figure 2.
Binding of Vpr and (52–96)Vpr to the double-stranded 21mer U5 ODN. Change in steady-state fluorescence anisotropy of fluorescein-labeled U5 ODN (10 nM) was monitored as a function of increasing concentrations of either full-length Vpr (filled squares) or (52–96)Vpr (filled triangles). The anisotropy value of labeled dsDNA alone recorded in our condition was close to 0.04 and was systematically subtracted from the anisotropy value of the DNA–protein complex (δ anisotropy). Addition of (1–51)Vpr did not lead to a change in anisotropy, indicating the absence of complex formation. Isotherm binding curves were fitted with Prism3.0 software.