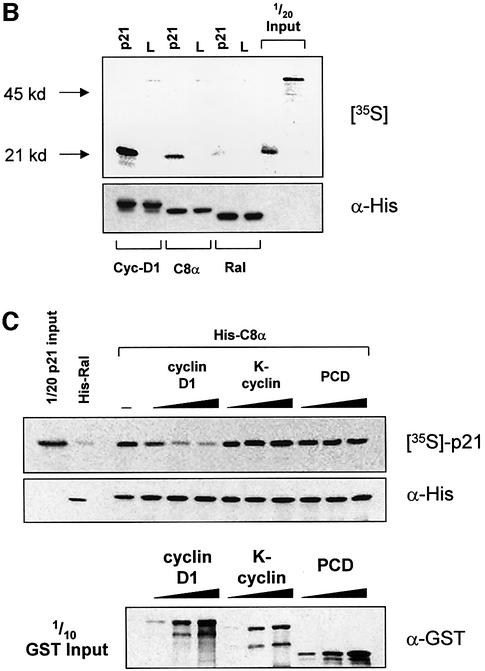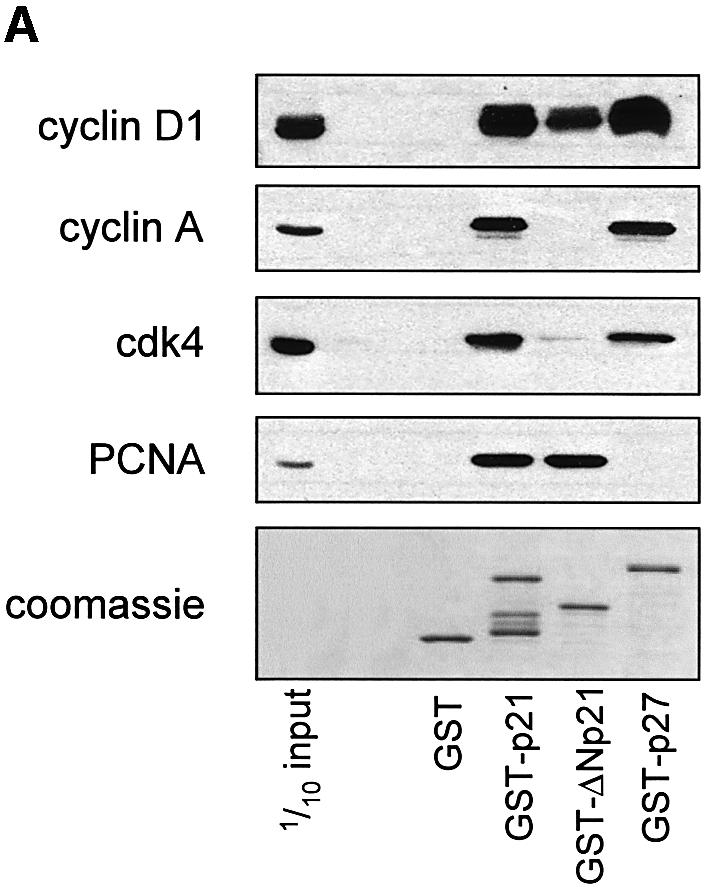
Fig. 5. Cyclin D1 binds to the p21 C-terminus and blocks the p21–C8α interaction. (A) Cyclin D1, but not cyclin A, binds to the Cy2 site of GST–p21. Ras-S3T3 extracts were incubated with the GST fusion proteins indicated, and captured complexes were western blotted for the p21-binding partners shown. A p21 mutant lacking the N-terminus was used (GST–ΔNp21) to determine which cyclins bind the C-terminal Cy2 site. (B) Association of p21 with C8α in vitro. IVTT-synthesized 35S-labelled p21 or luciferase control (L) were added to cyclin D1, C8α or Ral-His fusion proteins. Complexes were separated by SDS–PAGE, and His fusion-associated 35S-labelled proteins were detected by exposing membranes to autoradiography film. (C) Cyclin D1 competes with C8α for p21 binding. The assay described in (B) was repeated in the presence of 0, 2, 5 or 10 µg of cyclin D1, K-cyclin or GST–PCD fusions. Equal amounts of the different GST fusion proteins were added, as judged by western blot of one-tenth of the input.