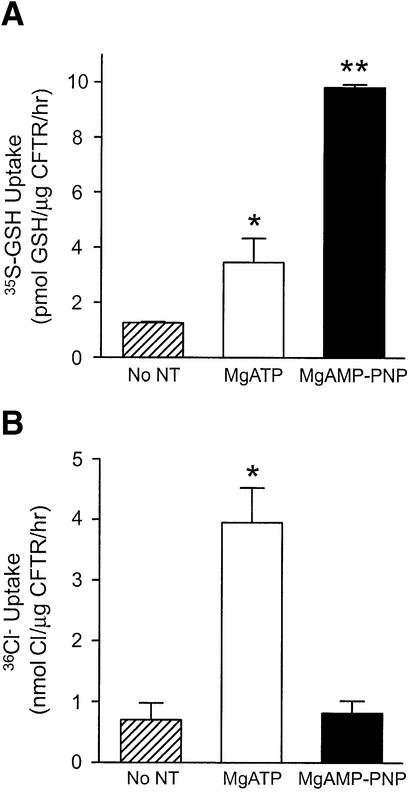
Fig. 6. Nucleotide-regulated GSH versus chloride flux by purified and reconstituted CFTR protein. (A) Proteoliposomes containing purified, reconstituted and phosphorylated CFTR were incubated with 20 nM [35S]GSH and 1 mM cold GSH at 33°C in CFTR transport buffer, in the presence of MgATP, MgAMP-PNP or no nucleotides for 60 min. GSH uptake values were normalized according to the amount of CFTR in each preparation. Values shown represent the mean activity (± SEM; n = 2–6). [35S]GSH uptake by different preparations was analyzed by one-way ANOVA, followed by Bonferroni’s multiple comparison test. The asterisks represent statistically significant differences in [35S]GSH flux relative to vesicles treated with no nucleotides (*P < 0.05; **P < 0.001). (B) 36Cl– flux in purified, reconstituted and phosphorylated CFTR was measured at 33°C in the presence of MgATP, MgAMP-PNP or no nucleotides, as previously described (Li, 1996). Flux values were normalized according to the amount of CFTR in each preparation. Values shown represent the mean activity (± SEM; n = 3). Chloride flux by different preparations was analyzed by one-way ANOVA, followed by Bonferroni’s multiple comparison test. The asterisks represent statistically significant differences in flux relative to vesicles treated with no nucleotides (*P < 0.001).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.