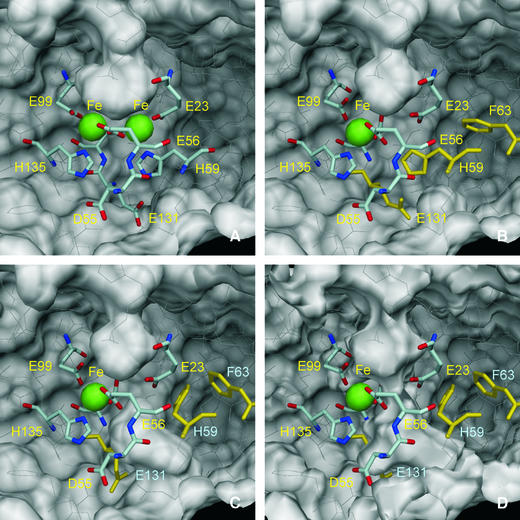
Fig. 5. The molecular surface of the Dd Bfr homodimer (PDB 1nfv), viewed from inside the molecule, near the di-iron centre. The iron atoms are represented as green spheres with 1.30 Å radius, and the residues that coordinate the iron atoms are drawn as sticks. Asp55 and Glu131, which form hydrogen bonds with iron-coordinating residues His135 and His59, respectively, are also drawn as sticks. Glu132, which bridges the di-iron centre, is partly hidden and is not labelled for clarity. The remaining residues in the Bfr homodimer are drawn as thin white lines. The outside surface of the homodimer is near the top of the figures, while the interior surface is near the bottom. A pocket in the external surface is clearly visible in all views. This pocket is larger in the ‘native’ (not shown) and reduced Bfr (A; PDB 1nf4) than in the oxidized ‘cycled’ structure (B; PDB 1nf6). (C) The hypothetical side-chain motions of His59, Phe63 and Glu131 (drawn in yellow) in the oxidized Bfr structure, which form a channel that allows access from the depleted iron site to the inside of the Bfr 24mer. A small pocket in the internal surface can be seen in (A) and (B) just below His59, which may be the precursor of this hypothetical access channel. In (A–C), the molecular surfaces were calculated with a 1.35 Å probe radius. In (D), the molecular surface was calculated with a 0.8 Å probe radius, and a channel that may allow a Fe2+ ion (radius 0.76 Å) to cross the protein shell is visible. The molecular surfaces were calculated with program MSMS (Sanner et al., 1996) and the figures were prepared with DINO (Philippsen, 2002).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.