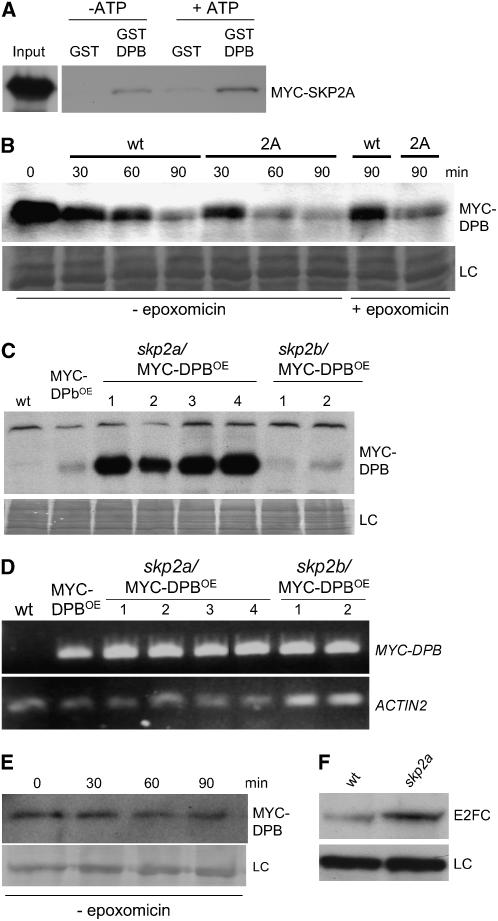
Figure 3.
SCFSKP2A Targets DPB/E2FC for Ubiquitin-Dependent Degradation.
(A) MYC-SKP2A interacts with GST-DPB but not with GST in pull-down experiments. The interaction was favored in the presence of high levels of ATP (+ATP) during the incubation.
(B) Time-course degradation of the MYC-DPB protein. Protein extracts from MYC-DPBOE plants were incubated with an extract of wild-type plants or with an extract of MYC-SKP2A–overexpressing plants (2A). Where indicated, the proteasome inhibitor (epoxomicin) was added. LC, loading control, corresponding to the same blot stained with Coomassie blue; min, incubation time in minutes.
(C) Protein extracts of wild type, MYC-DPBOE, and different lines (1 to 4) of skp2a/MYC-DPBOE and skp2b/MYC-DPBOE plants were examined by protein gel blot analysis using the anti-MYC antibody to detect MYC-DPB. LC, loading control, corresponding to the same blot stained with Coomassie blue.
(D) The expression level of the MYC-DPB transgene was analyzed by RT-PCR in the same plants used in (C). As a control, the level of the ACTIN2 gene was analyzed.
(E) Time-course degradation of the MYC-DPB protein. Protein extract from skp2a/MYC-DPBOE (line 1) plants was incubated for different times in a degradation assay. MYC-DPB was detected by immunoblotting. LC, unspecific cross-reacting protein used as a loading control.
(F) E2FC protein level in 5-d-old wild-type and skp2a seedlings.