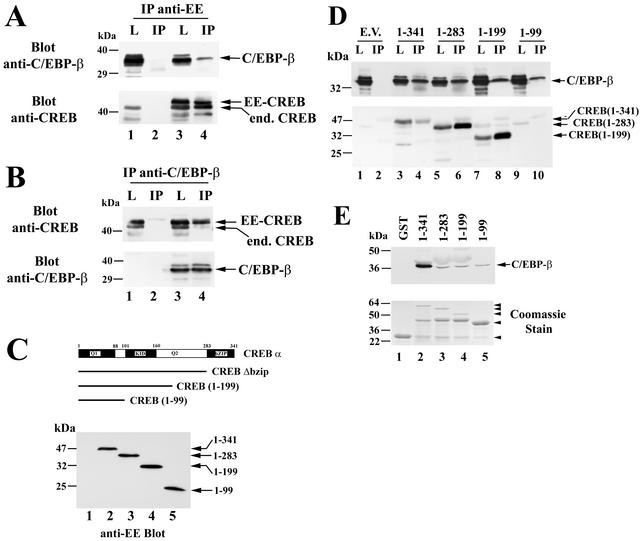
FIG. 11.
CREB and C/EBP-β association in vitro and in intact cells. (A) COS7 cells were electroporated with C/EBP-β vector (lanes 1 to 4) and either empty vector (lanes 1 and 2) or vector encoding EE epitope-tagged CREB (lanes 3 and 4). Cell lysates were subjected to immunoprecipitation with an anti-EE epitope antibody and washed immunoprecipitates (IP) or 5% of the input lysates (L) were analyzed by SDS-PAGE and Western blotting. Blots were developed with antibodies specific for C/EBP-β (upper panel) and reprobed with anti-CREB antibody (lower panel). Note the presence of endogenous CREB in cell lysates (lanes 1 and 3) as well as in immunoprecipitates containing EE-CREB, which heterodimerizes with endogenous CREB (lane 4); the lowest band most likely represents a proteolytic breakdown product, as it varied in intensity in different experiments. (B) COS7 cells were electroporated with EE-CREB (lanes 1 to 4) and either empty vector (lanes 1and 2) or vector encoding C/EBP-β (lanes 3 and 4). Immunoprecipitates (IP) were obtained with a C/EBP-β-specific antibody and analyzed side by side with 5% of input lysates (L) by Western blotting with antibodies specific for CREB (upper panel) and C/EBP-β (lower panel). Note that endogenous C/EBP-β in COS cells migrates with an apparent molecular mass of 42 to 45 kDa and is only visualized on longer exposures (not shown). (C) COS7 cells were cotransfected with C/EBP-β and either empty vector (lane 1) or vectors encoding EE epitope-tagged full-length CREB (lane 2), CREBΔbzip (lane 3), CREB truncated at amino acid 199 (lane 4), or CREB truncated at amino acid 99 (lane 5). Western blots were developed with anti-EE antibody. (D) Cell lysates of the COS7 cells described for panel C were subjected to immunoprecipitation with anti-EE antibody as described for panel A, and the immunoprecipitates (IP) as well as 5% of cell lysate input (L) were analyzed by Western blotting with an anti-C/EBP-β-specific antibody (upper panel) or a CREB-specific antibody (lower panel). Note that the anti-CREB antibody does not recognize the construct encoding amino acids 1 to 99 (lanes 9 and 10), but expression of the protein was demonstrated on the anti-EE blot shown in panel C. (E) GST (lane 1) or GST fusion proteins encoding full-length CREB or the indicated C-terminal CREB truncations (lanes 2 to 5) were bound to glutathione-agarose beads and incubated with purified Myc epitope-tagged C/EBP-β as described in Materials and Methods. Washed beads were analyzed by Western blotting with anti-Myc antibody to detect C/EBP-β (upper panel). An aliquot of the GST proteins was analyzed by SDS-PAGE and Coomassie staining (lower panel). The arrowheads indicate the migration of full-length proteins; in lanes 2 to 4, an ≈45-kDa proteolytic breakdown product is present.