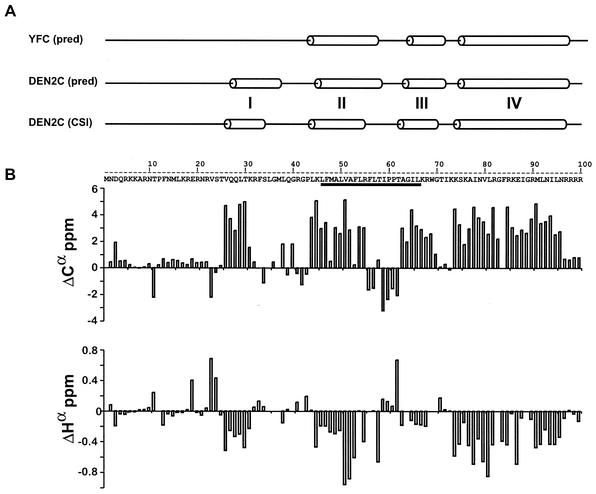
FIG. 4.
Flavivirus capsid is an alpha-helical protein. (A) Comparison of the secondary structure predictions of YFC and DEN2C. Secondary structure predictions for both proteins [YFC (pred) and DEN2C (pred)] were generated individually with ProteinPredict. The secondary structure predictions that are depicted are not sequence aligned. The secondary structure of DEN2C determined by a consensus of Cα, Cβ, Hα, and C′ chemical shift indexing [DEN2C (CSI)] is shown below the predicted secondary structures of YFC and DEN2C and is based on the data shown in panel B. Non-alpha-non-beta secondary structures are represented by a straight line. Alpha-helical secondary structures are represented by cylinders. The four alpha helices of DEN2C are indicated by the roman numerals I to IV. The underlined residues within the amino acid sequence of DEN2C indicate the internal hydrophobic sequence. The C-terminal signal sequence is not shown. (B) NMR analysis of the DEN C protein. The differences between observed sequence-specific chemical shift values and the corresponding predicted sequence corrected random-coil values were plotted against the corresponding residue number within DEN2C. Plots of Cα (top) and Hα (bottom) are shown.