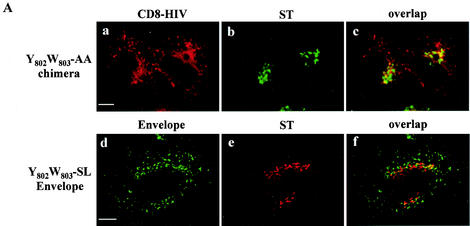
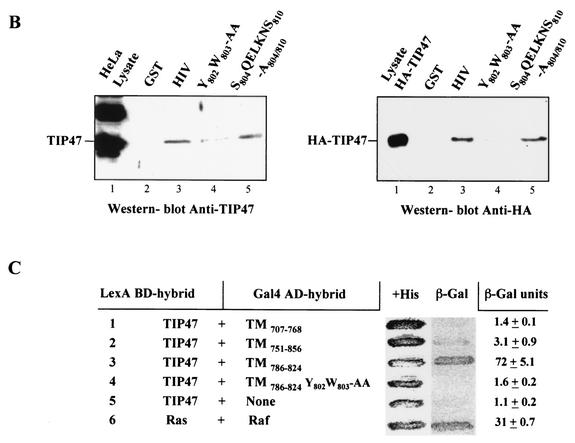
FIG.7.
Effect of mutations in the Y802W803 aromatic doublet on internalization of CD8-HIV chimera and Env glycoprotein. (A) HeLa cells expressing either mutated chimera CD8-HIV Y802W803-AA and ST (a to c) or Y802W803-SL-mutated Env and ST (d to f) were incubated at 0°C with the anti-CD8 Leu2A (a) or the b12 anti-Env (d) MAbs for 1 h and then returned to 37°C for 1 h. The cells were fixed, permeabilized, and labeled with anti-ST antibodies (b and e). Colocalization was examined by confocal microscopy (f). (B) Binding of the mutated cytosolic tail of Env to TIP47. HeLa cell lysates expressing endogenous TIP47 (left panel) or HA-TIP47 (right panel) were incubated with equal amounts of purified GST, GST-HIV, Y802W803-AA, and S804QELKNS810-A804/810 mutated GST-HIV. Bound materials were separated by SDS-PAGE, and TIP47 binding was analyzed by Western blotting with anti-TIP47 (left panel) or with anti-HA MAb (right panel). Crude lysates corresponding to 2 × 105 cells were also run as a control to detect TIP47 or HA-TIP47. (C) Analysis of the ability of the TMgp41 deletion mutant to interact with TIP47 in the two-hybrid assay. The yeast reporter strain L40 expressing the indicated hybrid protein pairs was analyzed for β-Gal expression. The TMgp41 deletion mutants indicated were fused to the Gal4 activation domain (Gal4AD). The TIP47 ORF was fused to the LexA BD. Ras and Raf proteins, which bind to each other efficiently, were used as positive controls.