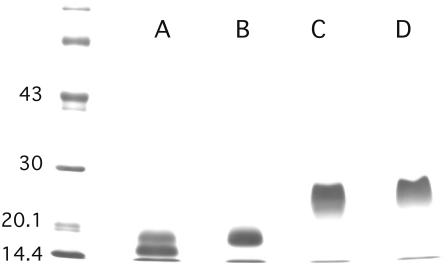
FIGURE 1.
PAGE of perlwapin. SDS-PAGE was done using 10–15% acrylamide gels which were stained with Coomassie blue. (Lanes A and B) Sample buffer containing mercaptoethanol as reducing agent; (lanes C and D) sample buffer without reducing agent. The sample run in lanes A and C contained 60–70% perlwapin molecules cleaved at the Tyr-99-Asp-100 bond, as estimated from sequence analysis. The sample run in lanes B and D showed no cleavage. The N-terminal and C-terminal fragments were held together by disulfide bonds in the cleaved sample if run under nonreducing conditions (lane D). The difference of ∼3000 Da between the major bands under reducing conditions (lanes A and B) is in good agreement with the loss of 35 amino acids in cleaved perlwapin (lane A). The position of molecular mass markers is indicated in kDa on the left.