TABLE 3.
Internal coordinates corresponding to lowest-energy minimum for Met-enkephalin, found in this work (columns 3–6) and previous work (last two columns)
| Torsion | E/2π | E/2 | E/3π | E/3 | 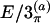 |
E/3(b) | |
|---|---|---|---|---|---|---|---|
| Tyr1 | χ1 | −179.8 | −172.6 | 59.9 | −173.2 | −174.2 | −173.2 |
| χ2 | 68.6 | −101.3 | 94.1 | −100.7 | −85.2 | −100.5 | |
| χ6 | −34.7 | 14.1 | −21.3 | 13.7 | 2.8 | 13.6 | |
| φ | −86.3 | −85.8 | 168.1 | −83.1 | −162.7 | −83.5 | |
| ψ | 153.7 | 156.2 | 0.9 | 155.8 | −41.7 | 155.8 | |
| ω | 180.0 | −176.9 | 180.0 | −177.1 | 180.0 | 177.2 | |
| Gly2 | φ | −161.5 | −154.5 | 126.8 | −154.2 | 65.8 | −154.3 |
| ψ | 71.1 | 83.7 | −21.2 | 85.8 | −87.0 | 86.0 | |
| ω | 180.0 | 168.6 | 180.0 | 168.5 | 180.0 | 168.5 | |
| Gly3 | φ | 64.1 | 83.7 | 83.7 | 83.0 | −157.3 | 83.0 |
| ψ | −93.5 | −73.9 | −61.6 | −75.0 | 34.9 | −75.1 | |
| ω | 180.0 | −170.1 | 180.0 | −170.0 | 180.0 | −169.9 | |
| Phe4 | χ1 | 179.8 | 58.8 | 58.6 | 58.9 | 52.4 | 58.8 |
| χ2 | −100.0 | −85.4 | 92.9 | −85.5 | −96.0 | −85.5 | |
| φ | −81.7 | −137.0 | −128.2 | −136.8 | −158.8 | −136.9 | |
| ψ | −29.2 | 19.3 | 18.8 | 19.1 | 159.5 | 19.1 | |
| ω | 180.0 | −174.1 | 180.0 | −174.1 | 180.0 | −174.1 | |
| Met5 | χ1 | −65.1 | 52.8 | 55.7 | 52.9 | −66.1 | 52.9 |
| χ2 | −179.2 | 175.3 | −178.6 | 175.3 | −179.6 | 175.3 | |
| χ3 | −179.3 | −179.8 | 177.0 | −179.9 | −179.9 | −179.9 | |
| χ4 | −179.9 | 61.4 | −179.3 | −178.6 | 60.1 | 61.4 | |
| φ | −80.7 | −163.6 | −162.1 | −163.4 | −82.4 | −163.5 | |
| ψ | 143.5 | 160.4 | 7.5 | 160.8 | 134.1 | 161.0 | |
| ω | 180.0 | −179.7 | 180.0 | −179.8 | 180.0 | −179.8 | |
| E (kcal/mol) | −10.72 | −12.91 | −10.90 | −12.43 | −10.85 | −11.71 |
In the columns labeled E/2 and E/3, the coordinates were obtained based on the ECEPP/2 and ECEPP/3 potentials, respectively. A subscript π indicates the fact that the peptide angles ω of the structure were fixed at 180° in the minimization process. The last two columns, labeled 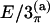 and E/3(b), contain the internal coordinates that can be found in Eisenmenger and Hansmann (11) and Androulakis et al. (13), respectively, for comparison.
and E/3(b), contain the internal coordinates that can be found in Eisenmenger and Hansmann (11) and Androulakis et al. (13), respectively, for comparison.
