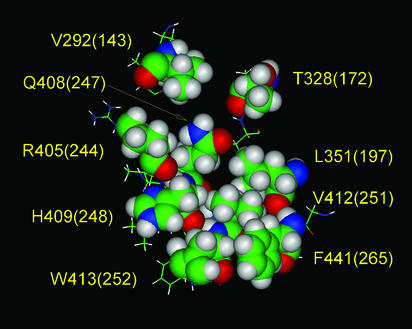
Figure 4.
The topography of the hydrophobic surface of the catalytic domain. The space-filling residues shown comprise a hydrophobic patch on the surface of the catalytic domain of PC3, which is postulated to interact with the P domain. An earlier model of this hydrophobic surface (37) was revised based on the results of the present mutagenesis study. The aliphatic portions of the side chains of R405 and W413 also may contribute to the hydrophobic patch, whereas I326 and S349 are excluded. Residue L410 was also excluded from this image because our modeling results indicated that the L410 side chain is directed toward the interior of the catalytic domain. The numbers in parentheses represent positions in subtilisin Carlsberg.