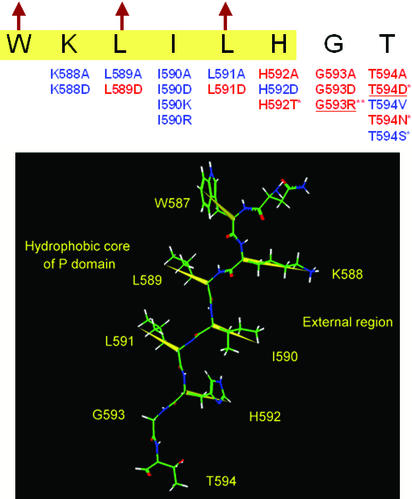
Figure 5.
Mutations near the carboxyl terminus of the P domain. ER-retained and tolerated mutations are shown by red and blue, respectively. Highlighted sequence (yellow) is in the region of the last β-strand of the P domain predicted by the Chou and Fasman method (43, 44), and arrows indicate hydrophobic residues that are directed toward the P domain's interior, according to the previous modeling study (37). The mutations marked by * have already been reported (33, 45), and underlined mutations were reconfirmed in this study. The spatial structure of this β-strand is given below, where the yellow vectors represent alternative directions of the side chains. W587, L589, and L591 are inside the protein and K588, I590, and H592 are outside. G593 forms a β-turn next to the β-strand.